The SrtA Sortase of Streptococcus agalactiae is required for cell wall anchoring of proteins containing the LPXTG motif, for adhesion to epithelial cells, and for colonization of the mouse intestine
- PMID: 15908360
- PMCID: PMC1111822
- DOI: 10.1128/IAI.73.6.3342-3350.2005
The SrtA Sortase of Streptococcus agalactiae is required for cell wall anchoring of proteins containing the LPXTG motif, for adhesion to epithelial cells, and for colonization of the mouse intestine
Abstract
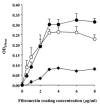
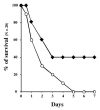
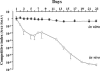
Streptococcus agalactiae (group B streptococcus [GBS]) is the leading cause of neonatal pneumonia, sepsis, and meningitis. An in silico genome analysis indicated that GBS strain NEM316 encodes 35 proteins containing an LPXTG motif which are thought to be covalently linked to the peptidoglycan by an enzyme called sortase. The role of these cell wall-anchored proteins in GBS pathogenesis was evaluated on a global level by inactivating the srtA gene. This gene encodes the major sortase SrtA that anchors most of the LPXTG-containing proteins. We chose the C5a peptidase (ScpB) and Alp2, an abundant immunogenic protein, as prototypical LPXTG-containing proteins. As expected, the SrtA knockout mutant was unable to anchor the C5a peptidase (ScpB) and Alp2 to the cell wall. Complementation with plasmid-borne srtA inserted into the chromosome restored the correct surface localization of both ScpB and Alp2. Interestingly, the SrtA mutant was impaired for binding to the major extracellular matrix components fibronectin and fibrinogen and displayed a significant reduction in adherence to human (A549, HeLa, and Caco-2) and murine (L2) epithelial cells compared to the parental wild-type strain. Surprisingly, the inactivation of srtA had no effect on the virulence of the type III strain of GBS in a neonatal rat model (measured by the 50% lethal dose and lung colonization) but strongly impaired the capacity of the strain to colonize the intestines of gnotobiotic mice in a competition assay. These results demonstrate that LPXTG-containing proteins are involved in cell adhesion and GBS persistence in vivo.
Figures
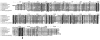


References
-
- Bierne, H., S. K. Mazmanian, M. Trost, M. G. Pucciarelli, G. Liu, P. Dehoux, L. Jansch, F. Garcia-del Portillo, O. Schneewind, and P. Cossart. 2002. Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol. Microbiol. 43:869-881. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

