Increased sensitivity to staphylococcal enterotoxin B following adenoviral infection
- PMID: 15908364
- PMCID: PMC1111844
- DOI: 10.1128/IAI.73.6.3375-3384.2005
Increased sensitivity to staphylococcal enterotoxin B following adenoviral infection
Abstract
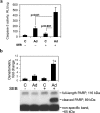
Staphylococcal enterotoxin B induces toxic shock and is a major virulence factor of staphylococcal diseases. We examined the effects of systemic adenoviral infection on responses to staphylococcal enterotoxin B in a murine model. We found that adenoviral infection markedly increases the severity of liver injury following exposure to staphylococcal enterotoxin B without d-galactosamine sensitization. In adenovirus-infected mice, staphylococcal enterotoxin B triggered a more profound hypothermia and increased apoptosis in the liver. Consistent with these observations, we also found that adenoviral infection primed for an increased production of gamma interferon in vivo and in vitro following stimulation with staphylococcal enterotoxin B. Gamma-interferon-knockout mice did not show increased sensitivity to staphylococcal enterotoxin B following adenoviral infection. These data suggest that a preexisting viral infection primes mice for subsequent staphylococcal enterotoxin B exposure, possibly via a gamma-interferon-mediated mechanism.
Figures
Similar articles
-
T cells made deficient in interleukin-2 production by exposure to staphylococcal enterotoxin B in vivo are primed for interferon-gamma and interleukin-10 secretion.Eur J Immunol. 1995 May;25(5):1148-53. doi: 10.1002/eji.1830250503. Eur J Immunol. 1995. PMID: 7774618
-
Chronic Staphylococcal enterotoxin B and lipopolysaccharide induce a bimodal pattern of hepatic dysfunction and injury.Crit Care Med. 2003 Apr;31(4):1154-9. doi: 10.1097/01.CCM.0000060004.85054.F2. Crit Care Med. 2003. PMID: 12682487
-
Staphylococcal enterotoxin B toxicity in BALB/c mice: effect on T-cells, plasma cytokine levels and biochemical markers.FEMS Immunol Med Microbiol. 1995 Apr;11(2):91-7. doi: 10.1111/j.1574-695X.1995.tb00094.x. FEMS Immunol Med Microbiol. 1995. PMID: 7640677
-
Role of CD44 and its v7 isoform in staphylococcal enterotoxin B-induced toxic shock: CD44 deficiency on hepatic mononuclear cells leads to reduced activation-induced apoptosis that results in increased liver damage.Infect Immun. 2005 Jan;73(1):50-61. doi: 10.1128/IAI.73.1.50-61.2005. Infect Immun. 2005. PMID: 15618140 Free PMC article.
-
Tumor necrosis factor-induced hepatic DNA fragmentation as an early marker of T cell-dependent liver injury in mice.Gastroenterology. 1995 Jul;109(1):166-76. doi: 10.1016/0016-5085(95)90282-1. Gastroenterology. 1995. PMID: 7797015
Cited by
-
Severe acute hepatitis of unknown etiology in children in 2022: A Narrative Review.New Microbes New Infect. 2023 Jan 21;51:101087. doi: 10.1016/j.nmni.2023.101087. eCollection 2023 Jan. New Microbes New Infect. 2023. PMID: 36755794 Free PMC article. Review.
-
Solved the enigma of pediatric severe acute hepatitis of unknown origin?Front Cell Infect Microbiol. 2023 Sep 21;13:1175996. doi: 10.3389/fcimb.2023.1175996. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37808908 Free PMC article. Review.
-
Impact of Staphylococcus aureus on pathogenesis in polymicrobial infections.Infect Immun. 2014 Jun;82(6):2162-9. doi: 10.1128/IAI.00059-14. Epub 2014 Mar 18. Infect Immun. 2014. PMID: 24643542 Free PMC article. Review.
-
Acute severe hepatitis outbreak in children: A perfect storm. What do we know, and what questions remain?Front Pharmacol. 2022 Nov 25;13:1062408. doi: 10.3389/fphar.2022.1062408. eCollection 2022. Front Pharmacol. 2022. PMID: 36506522 Free PMC article. Review.
-
Severe acute hepatitis in children: investigate SARS-CoV-2 superantigens.Lancet Gastroenterol Hepatol. 2022 Jul;7(7):594-595. doi: 10.1016/S2468-1253(22)00166-2. Epub 2022 May 14. Lancet Gastroenterol Hepatol. 2022. PMID: 35576952 Free PMC article. No abstract available.
References
-
- Anderson, M. R., and M. Tary-Lehmann. 2001. Staphylococcal enterotoxin-B-induced lethal shock in mice is T-cell-dependent, but disease susceptibility is defined by the non-T-cell compartment. Clin. Immunol. 98:85-94. - PubMed
-
- Aroeira, L. S., and A. C. Martinez. 1999. The role of IL-4 in the staphylococcal enterotoxin B-triggered immune response: increased susceptibility to shock and deletion of CD8Vβ8+ T cells in IL-4 knockout mice. Eur. J. Immunol. 29:1397-1405. - PubMed
-
- Barraza, E. M., S. L. Ludwig, J. C. Gaydos, and J. F. Brundage. 1999. Reemergence of adenovirus type 4 acute respiratory disease in military trainees: report of an outbreak during a lapse in vaccination. J. Infect. Dis. 179:1531-1533. - PubMed
-
- Cameron, S. B., M. C. Nawijn, W. W. Kum, H. F. Savelkoul, and A. W. Chow. 2001. Regulation of helper T cell responses to staphylococcal superantigens. Eur. Cytokine Netw. 12:210-222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

