Combining anthrax vaccine and therapy: a dominant-negative inhibitor of anthrax toxin is also a potent and safe immunogen for vaccines
- PMID: 15908368
- PMCID: PMC1111877
- DOI: 10.1128/IAI.73.6.3408-3414.2005
Combining anthrax vaccine and therapy: a dominant-negative inhibitor of anthrax toxin is also a potent and safe immunogen for vaccines
Abstract
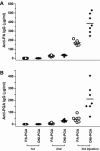
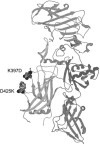
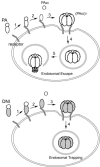
Anthrax is caused by the unimpeded growth of Bacillus anthracis in the host and the secretion of toxins. The currently available vaccine is based on protective antigen (PA), a central component of anthrax toxin. Vaccination with PA raises no direct immune response against the bacilli and, being a natural toxin component, PA might be hazardous when used immediately following exposure to B. anthracis. Thus, we have sought to develop a vaccine or therapeutic agent that is safe and eliminates both secreted toxins and bacilli. To that end, we have previously developed a dually active vaccine by conjugating the capsular poly-gamma-d-glutamate (PGA) with PA to elicit the production of antibodies specific for both bacilli and toxins. In the present report, we describe the improved potency of anthrax vaccines through the use of a dominant-negative inhibitory (DNI) mutant to replace PA in PA or PA-PGA vaccines. When tested in mice, DNI alone is more immunogenic than PA, and DNI-PGA conjugate elicits significantly higher levels of antibodies against PA and PGA than PA-PGA conjugate. To explain the enhanced immunogenicity of DNI, we propose that the two point mutations in DNI may have improved epitopes of PA allowing better antigen presentation to helper T cells. Alternatively, these mutations may enhance the immunological processing of PA by altering endosomal trafficking of the toxin in antigen-presenting cells. Because DNI has previously been demonstrated to inhibit anthrax toxin, postexposure use of DNI-based vaccines, including conjugate vaccines, may provide improved immunogenicity and therapeutic activity simultaneously.
Figures

References
-
- Anonymous. 2000. Use of anthrax vaccine in the United States. Morb. Mortal. Wkly. Rep. 49(RR-15):1-20. - PubMed
-
- Benson, E. L., P. D. Huynh, A. Finkelstein, and R. J. Collier. 1998. Identification of residues lining the anthrax protective antigen channel. Biochemistry 37:3941-3948. - PubMed
-
- Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225-229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

