Impairment of protective immunity to blood-stage malaria by concurrent nematode infection
- PMID: 15908382
- PMCID: PMC1111846
- DOI: 10.1128/IAI.73.6.3531-3539.2005
Impairment of protective immunity to blood-stage malaria by concurrent nematode infection
Abstract
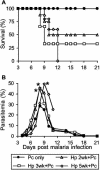
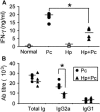
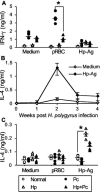
Helminthiases, which are highly prevalent in areas where malaria is endemic, have been shown to modulate or suppress the immune response to unrelated antigens or pathogens. In this study, we established a murine model of coinfection with a gastrointestinal nematode parasite, Heligmosomoides polygyrus, and the blood-stage malaria parasite Plasmodium chabaudi AS in order to investigate the modulation of antimalarial immunity by concurrent nematode infection. Chronic infection with the nematode for 2, 3, or 5 weeks before P. chabaudi AS infection severely impaired the ability of C57BL/6 mice to control malaria, as demonstrated by severe mortality and significantly increased malaria peak parasitemia levels. Coinfected mice produced significantly lower levels of gamma interferon (IFN-gamma) during P. chabaudi AS infection than mice infected with malaria alone. Concurrent nematode infection also suppressed production of type 1-associated, malaria-specific immunoglobulin G2a. Mice either infected with the nematode alone or coinfected with the nematode and malaria had high transforming growth factor beta1 (TGF-beta1) levels, and concurrent nematode and malaria infections resulted in high levels of interleukin-10 in vivo. Splenic CD11c(+) dendritic cells (DC) from mice infected with malaria alone and coinfected mice showed similarly increased expression of CD40, CD80, and CD86, but DC from coinfected mice were unable to induce CD4(+) T-cell proliferation and optimal IFN-gamma production in response to the malaria antigen in vitro. Importantly, treatment of nematode-infected mice with an anthelmintic drug prior to malaria infection fully restored protective antimalarial immunity and reduced TGF-beta1 levels. These results demonstrate that concurrent nematode infection strongly modulates multiple aspects of immunity to blood-stage malaria and consequently impairs the development of protective antimalarial immunity.
Figures



Similar articles
-
Reduced protective efficacy of a blood-stage malaria vaccine by concurrent nematode infection.Infect Immun. 2006 Apr;74(4):2138-44. doi: 10.1128/IAI.74.4.2138-2144.2006. Infect Immun. 2006. PMID: 16552043 Free PMC article.
-
Concurrent infection with Heligmosomoides polygyrus suppresses anti-Plasmodium yoelii protection partially by induction of CD4(+)CD25(+)Foxp3(+) Treg in mice.Eur J Immunol. 2009 Oct;39(10):2822-30. doi: 10.1002/eji.200939433. Eur J Immunol. 2009. PMID: 19728313
-
Modulation of malaria-induced immunopathology by concurrent gastrointestinal nematode infection in mice.Int J Parasitol. 2009 Dec;39(14):1525-32. doi: 10.1016/j.ijpara.2009.08.007. Epub 2009 Aug 27. Int J Parasitol. 2009. PMID: 19716371
-
Dendritic cells, pro-inflammatory responses, and antigen presentation in a rodent malaria infection.Immunol Rev. 2004 Oct;201:35-47. doi: 10.1111/j.0105-2896.2004.00182.x. Immunol Rev. 2004. PMID: 15361231 Review.
-
The response of CD4+ T cells to Plasmodium chabaudi chabaudi.Immunol Rev. 1989 Dec;112:71-94. doi: 10.1111/j.1600-065x.1989.tb00553.x. Immunol Rev. 1989. PMID: 2575075 Review.
Cited by
-
Novel exosome-targeted T-cell-based vaccine counteracts T-cell anergy and converts CTL exhaustion in chronic infection via CD40L signaling through the mTORC1 pathway.Cell Mol Immunol. 2017 Jun;14(6):529-545. doi: 10.1038/cmi.2016.23. Epub 2016 Jun 6. Cell Mol Immunol. 2017. PMID: 27264687 Free PMC article.
-
Modulation of dendritic cell function and immune response by cysteine protease inhibitor from murine nematode parasite Heligmosomoides polygyrus.Immunology. 2013 Apr;138(4):370-81. doi: 10.1111/imm.12049. Immunology. 2013. PMID: 23240853 Free PMC article.
-
Modelling co-infection with malaria and lymphatic filariasis.PLoS Comput Biol. 2013;9(6):e1003096. doi: 10.1371/journal.pcbi.1003096. Epub 2013 Jun 13. PLoS Comput Biol. 2013. PMID: 23785271 Free PMC article.
-
Impact of Schistosoma mansoni on malaria transmission in Sub-Saharan Africa.PLoS Negl Trop Dis. 2014 Oct 16;8(10):e3234. doi: 10.1371/journal.pntd.0003234. eCollection 2014 Oct. PLoS Negl Trop Dis. 2014. PMID: 25329403 Free PMC article.
-
Down Regulation of the TCR Complex CD3ζ-Chain on CD3+ T Cells: A Potential Mechanism for Helminth-Mediated Immune Modulation.Front Immunol. 2015 Feb 18;6:51. doi: 10.3389/fimmu.2015.00051. eCollection 2015. Front Immunol. 2015. PMID: 25741337 Free PMC article.
References
-
- Allen, J. E., and A. S. MacDonald. 1998. Profound suppression of cellular proliferation mediated by the secretions of nematodes. Parasite Immunol. 20:241-247. - PubMed
-
- Behnke, J. M., and M. Robinson. 1985. Genetic control of immunity to Nematospiroides dubius: a 9-day anthelmintic abbreviated immunizing regime which separates weak and strong responder strains of mice. Parasite Immunol. 7:235-253. - PubMed
-
- Borkow, G., Z. Weisman, Q. Leng, M. Stein, A. Kalinkovich, D. Wolday, and Z. Bentwich. 2001. Helminths, human immunodeficiency virus and tuberculosis. Scand. J. Infect. Dis. 33:568-571. - PubMed
-
- Buendia, A. J., P. G. Fallon, L. Del Rio, N. Ortega, M. R. Caro, M. C. Gallego, and J. Salinas. 2002. Previous infection with the nematode Nippostrongylus brasiliensis alters the immune specific response against Chlamydophila abortus infection. Microb. Pathog. 33:7-15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

