Vaccination of cattle with a CpG oligodeoxynucleotide-formulated mycobacterial protein vaccine and Mycobacterium bovis BCG induces levels of protection against bovine tuberculosis superior to those induced by vaccination with BCG alone
- PMID: 15908383
- PMCID: PMC1111861
- DOI: 10.1128/IAI.73.6.3540-3546.2005
Vaccination of cattle with a CpG oligodeoxynucleotide-formulated mycobacterial protein vaccine and Mycobacterium bovis BCG induces levels of protection against bovine tuberculosis superior to those induced by vaccination with BCG alone
Abstract
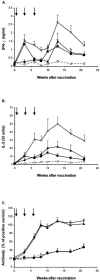
The development of a subunit protein vaccine for bovine tuberculosis which could be used either in combination with Mycobacterium bovis BCG (to improve the efficacy of that vaccine) or alone would offer significant advantages over currently available strategies. A study was conducted with cattle to determine the protective efficacy of a strategy based on concurrent immunization with an M. bovis culture filtrate (CFP) vaccine and BCG compared to vaccination with either vaccine alone. One group of calves (10 animals per group) was vaccinated subcutaneously with CFP formulated with Emulsigen and combined with a CpG oligodeoxynucleotide (ODN). A second group was vaccinated with both the CFP vaccine and BCG injected at adjacent sites (CFP-BCG). One further group was vaccinated subcutaneously with BCG, while another group served as nonvaccinated control animals. Vaccination with CFP-BCG induced levels of antigen-specific gamma interferon (IFN-gamma) and interleukin-2 (IL-2) in whole-blood cultures that were higher than those induced by vaccination with BCG alone. The combination of CFP and BCG did not enhance the production of antibodies to M. bovis CFP compared to vaccination with CFP alone. Vaccination with CFP alone led to delayed antigen-specific IFN-gamma and IL-2 responses. Vaccination with CFP-BCG induced a high level of protection against an intratracheal challenge with virulent M. bovis, based on a significant enhancement of six pathological and microbiological parameters of protection compared with the nonvaccinated group. In contrast, vaccination with BCG alone induced a significant enhancement of protection in only one parameter, while CFP alone induced no protection. These results suggest that a combination of a CpG ODN-formulated protein vaccine and BCG offers better protection against bovine tuberculosis than does BCG alone.
Figures
References
-
- Aronson, N. E., M. Santosham, G. W. Comstock, R. S. Howard, L. H. Moulton, E. R. Rhoades, and L. H. Harrison. 2004. Long-term efficacy of BCG vaccine in American Indians and Alaska natives: a 60-year follow-up study. JAMA 291:2086-2091. - PubMed
-
- Brandt, L., J. Feino Cunha, A. Weinreich Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

