Peripheral blood and pleural fluid mononuclear cell responses to low-molecular-mass secretory polypeptides of Mycobacterium tuberculosis in human models of immunity to tuberculosis
- PMID: 15908384
- PMCID: PMC1111830
- DOI: 10.1128/IAI.73.6.3547-3558.2005
Peripheral blood and pleural fluid mononuclear cell responses to low-molecular-mass secretory polypeptides of Mycobacterium tuberculosis in human models of immunity to tuberculosis
Erratum in
- Infect Immun. 2005 Dec;73(12):8456
Abstract
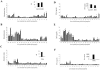
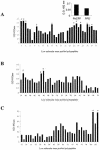
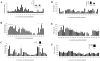
A total of 104 polypeptides were purified from the low-molecular-mass secretory proteome of Mycobacterium tuberculosis H(37)Rv using a combination of anion exchange column chromatography and high resolution preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by electroelution. The goal of this study was to identify polypeptides from a low-molecular-mass secretory proteome recognized by human subjects infected with M. tuberculosis and to ascertain the differences in specificity of antigen recognition by the peripheral blood mononuclear cells (PBMCs) and pleural fluid mononuclear cells (PFMCs) of these individuals. The study identified CFP-8 (Rv0496), CFP-11 (Rv2433c), CFP-14.5 (Rv2445c), and CFP-31 (Rv0831c) as novel T-cell antigens apart from previously characterized ESAT-6, TB10.4, CFP10, GroES, MTSP14, MTSP17, CFP21, MPT64, Ag85A, and Ag85B on the basis of recognition by PBMCs of tuberculosis contacts and treated tuberculosis patients. Further, polypeptides prominently recognized by PFMCs of tuberculous pleurisy patients were the same as those recognized by PBMCs of healthy contacts and treated tuberculosis patients. The results of our study indicate the homogeneity of antigenic target recognition by lymphocytes at the site of infection and at the periphery in the human subjects studied and the need to evaluate these antigenic targets as components of future antituberculous vaccines.
Figures


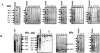
Similar articles
-
Lung and blood mononuclear cell responses of tuberculosis patients to mycobacterial proteins.Eur Respir J. 2007 Feb;29(2):337-46. doi: 10.1183/09031936.00111205. Epub 2006 Nov 1. Eur Respir J. 2007. PMID: 17079254
-
ESAT-6- and CFP-10-specific Th1, Th22 and Th17 cells in tuberculous pleurisy may contribute to the local immune response against Mycobacterium tuberculosis infection.Scand J Immunol. 2011 Apr;73(4):330-7. doi: 10.1111/j.1365-3083.2011.02512.x. Scand J Immunol. 2011. PMID: 21223348
-
[Detection of specific interferon-gamma-secreting T cell response to Mycobacterium tuberculosis RD1-encoded antigens in pleural effusions, ascites, and cerebrospinal fluid].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009 Aug;31(4):438-42. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009. PMID: 19771730 Chinese.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
[Some problems concerning local cellular immunity in tuberculosis].Kekkaku. 1995 Oct;70(10):595-600. Kekkaku. 1995. PMID: 8523853 Review. Japanese.
Cited by
-
Expression of the ARPC4 subunit of human Arp2/3 severely affects mycobacterium tuberculosis growth and suppresses immunogenic response in murine macrophages.PLoS One. 2013 Jul 22;8(7):e69949. doi: 10.1371/journal.pone.0069949. Print 2013. PLoS One. 2013. PMID: 23894563 Free PMC article.
-
The role of the novel exopolyphosphatase MT0516 in Mycobacterium tuberculosis drug tolerance and persistence.PLoS One. 2011;6(11):e28076. doi: 10.1371/journal.pone.0028076. Epub 2011 Nov 21. PLoS One. 2011. PMID: 22132215 Free PMC article.
-
Immunoproteomic identification of human T cell antigens of Mycobacterium tuberculosis that differentiate healthy contacts from tuberculosis patients.Mol Cell Proteomics. 2010 Mar;9(3):538-49. doi: 10.1074/mcp.M900299-MCP200. Epub 2009 Dec 22. Mol Cell Proteomics. 2010. PMID: 20031926 Free PMC article.
-
HigA2 (Rv2021c) Is a Transcriptional Regulator with Multiple Regulatory Targets in Mycobacterium tuberculosis.Microorganisms. 2024 Jun 20;12(6):1244. doi: 10.3390/microorganisms12061244. Microorganisms. 2024. PMID: 38930627 Free PMC article.
-
Cutinase-like proteins of Mycobacterium tuberculosis: characterization of their variable enzymatic functions and active site identification.FASEB J. 2009 Jun;23(6):1694-704. doi: 10.1096/fj.08-114421. Epub 2009 Feb 18. FASEB J. 2009. PMID: 19225166 Free PMC article.
References
-
- Andersen, P., and I. Heron. 1993. Simultaneous electroelution of whole SDS-polyacrylamide gels for the direct cellular analysis of complex protein mixtures. J. Immunol. Methods 161:29-39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

