Opsonized virulent Brucella abortus replicates within nonacidic, endoplasmic reticulum-negative, LAMP-1-positive phagosomes in human monocytes
- PMID: 15908400
- PMCID: PMC1111828
- DOI: 10.1128/IAI.73.6.3702-3713.2005
Opsonized virulent Brucella abortus replicates within nonacidic, endoplasmic reticulum-negative, LAMP-1-positive phagosomes in human monocytes
Abstract
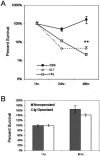
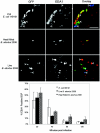
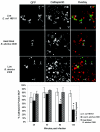
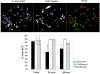
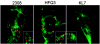
Cells in the Brucella spp. are intracellular pathogens that survive and replicate within host monocytes. Brucella maintains persistent infections in animals despite the production of high levels of anti-Brucella-specific antibodies. To determine the effect of antibody opsonization on the ability of Brucella to establish itself within monocytes, the intracellular trafficking of virulent Brucella abortus 2308 and attenuated hfq and bacA mutants was followed in the human monocytic cell line THP-1. Early trafficking events of B. abortus 2308-containing phagosomes (BCP) were indistinguishable from those seen for control particles (heat-killed B. abortus 2308, live Escherichia coli HB101, or latex beads). All phagosomes transiently communicated the early-endosomal compartment and rapidly matured into LAMP-1(+), cathepsin D(+), and acidic phagosomes. By 2 h postinfection, however, the number of cathepsin D(+) BCP was significantly lower for live B. abortus 2308-infected cells than for either Brucella mutant strains or control particles. B. abortus 2308 persisted within these cathepsin D(-), LAMP-1(+), and acidic vesicles; however, at the onset of intracellular replication, the numbers of acidic B. abortus 2308 BCP decreased while remaining cathepsin D(-) and LAMP-1(+). In contrast to B. abortus 2308, the isogenic hfq and bacA mutants remained in acidic, LAMP-1(+) phagosomes and failed to initiate intracellular replication. Notably, markers specific for the host endoplasmic reticulum were absent from the BCPs throughout the course of the infection. Thus, opsonized B. abortus in human monocytes survives within phagosomes that remain in the endosomal pathway and replication of virulent B. abortus 2308 within these vesicles corresponds with an increase in intraphagosomal pH.
Figures

References
-
- Baldwin, C. L., and M. Parent. 2002. Fundamentals of host immune response against Brucella abortus: what the mouse model has revealed about control of infection. Vet. Microbiol. 90:367-382. - PubMed
-
- Baldwin, C. L., and A. J. Winter. 1994. Macrophages and Brucella. Immunol. Ser. 60:363-380. - PubMed
-
- Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevieille, J. P. Liautard, M. Ramuz, and D. O'Callaghan. 2002. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. USA 99:1544-1549. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

