CD4+ T cells play a dominant role in protection against New World leishmaniasis induced by vaccination with the P-4 amastigote antigen
- PMID: 15908422
- PMCID: PMC1111832
- DOI: 10.1128/IAI.73.6.3823-3827.2005
CD4+ T cells play a dominant role in protection against New World leishmaniasis induced by vaccination with the P-4 amastigote antigen
Abstract
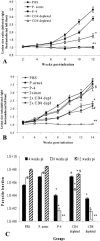
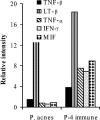
Immunodepletion studies of P-4-vaccinated mice indicate that CD4+ and not CD8+ T cells are critical for protection against Leishmania pifanoi (Leishmania mexicana complex). Although a moderate CD8+ T-cell response is elicited by vaccination, CD4+ T cells are the dominant responding population in vitro and at the cutaneous site of infection. These protective T cells produce gamma interferon (IFN-gamma), macrophage migration inhibitory factor (MIF), and tumor necrosis factor/lymphotoxin (TNF/LT), each of which significantly contributed to intracellular parasite destruction in vitro. These results indicate that a singular CD4+ T-cell response (IFN-gamma, MIF, and/or LT/TNF) can provide protection against New World cutaneous leishmaniasis.
Figures


Similar articles
-
Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection.Infect Immun. 2003 Jun;71(6):3172-82. doi: 10.1128/IAI.71.6.3172-3182.2003. Infect Immun. 2003. PMID: 12761096 Free PMC article.
-
Vaccination with a plasmid DNA cocktail encoding the nucleosomal histones of Leishmania confers protection against murine cutaneous leishmaniosis.Vaccine. 2004 Sep 28;22(29-30):3865-76. doi: 10.1016/j.vaccine.2004.04.015. Vaccine. 2004. PMID: 15364433
-
MVA-LACK as a safe and efficient vector for vaccination against leishmaniasis.Microbes Infect. 2006 Mar;8(3):810-22. doi: 10.1016/j.micinf.2005.10.004. Epub 2006 Jan 13. Microbes Infect. 2006. PMID: 16504562
-
CD4+ T Cell-Mediated Immunity against the Phagosomal Pathogen Leishmania: Implications for Vaccination.Trends Parasitol. 2019 Jun;35(6):423-435. doi: 10.1016/j.pt.2019.04.002. Epub 2019 May 9. Trends Parasitol. 2019. PMID: 31080088 Review.
-
Immunology of leishmaniasis.Curr Opin Immunol. 1992 Aug;4(4):413-8. doi: 10.1016/s0952-7915(06)80032-4. Curr Opin Immunol. 1992. PMID: 1356346 Review.
Cited by
-
Role of Host and Parasite MIF Cytokines during Leishmania Infection.Trop Med Infect Dis. 2020 Mar 20;5(1):46. doi: 10.3390/tropicalmed5010046. Trop Med Infect Dis. 2020. PMID: 32244916 Free PMC article. Review.
-
Macrophage migration inhibitory factor in protozoan infections.J Parasitol Res. 2012;2012:413052. doi: 10.1155/2012/413052. Epub 2012 Feb 9. J Parasitol Res. 2012. PMID: 22496958 Free PMC article.
-
Identification of Potential MHC Class-II-Restricted Epitopes Derived from Leishmania donovani Antigens by Reverse Vaccinology and Evaluation of Their CD4+ T-Cell Responsiveness against Visceral Leishmaniasis.Front Immunol. 2017 Dec 14;8:1763. doi: 10.3389/fimmu.2017.01763. eCollection 2017. Front Immunol. 2017. PMID: 29312304 Free PMC article.
-
Vaccination in Leishmaniasis: A Review Article.Iran Biomed J. 2022 Jan 1;26(1):1-35. doi: 10.52547/ibj.26.1.35. Iran Biomed J. 2022. PMID: 34952558 Free PMC article.
-
Macrophage migration inhibitory factor (MIF): a key player in protozoan infections.Int J Biol Sci. 2011;7(9):1239-56. doi: 10.7150/ijbs.7.1239. Epub 2011 Oct 25. Int J Biol Sci. 2011. PMID: 22110378 Free PMC article. Review.
References
-
- Denkinger, C. M., M. Denkinger, J. J. Kort, C. Metz, and T. G. Forsthuber. 2003. In vivo blockade of macrophage migration inhibitory factor ameliorates acute experimental autoimmune encephalomyelitis by impairing the homing of encephalitogenic T cells to the central nervous system. J. Immunol. 170:1274-1282. - PubMed
-
- Jin, F. S., R. J. Youle, V. G. Johnson, J. Shiloach, R. Fass, D. L. Longo, and S. H. Bridges. 1991. Suppression of the immune response to immunotoxins with anti-CD4 monoclonal antibodies. J. Immunol. 146:1806-1811. - PubMed
-
- Juttner, S., J. Bernhagen, C. N. Metz, M. Rollinghoff, R. Bucala, and A. Gessner. 1998. Migration inhibitory factor induces killing of Leishmania major by macrophages: dependence on reactive nitrogen intermediates and endogenous TNF-alpha. J. Immunol. 161:2383-2390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

