Keratocan expression of murine keratocytes is maintained on amniotic membrane by down-regulating transforming growth factor-beta signaling
- PMID: 15908433
- PMCID: PMC1282515
- DOI: 10.1074/jbc.M409567200
Keratocan expression of murine keratocytes is maintained on amniotic membrane by down-regulating transforming growth factor-beta signaling
Abstract
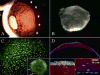
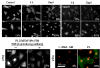
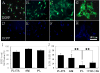
Keratocytes in the corneal stroma express keratan sulfate-containing proteoglycans including cornea-specific keratocan. On plastic dishes, human, bovine, and rabbit keratocytes lose their characteristic dendritic morphology and keratocan expression when cultured in serum-containing media. Herein, we demonstrated that murine keratocytes also acquired a fibroblastic shape and lost keratocan expression after first passage when cultured on plastic in the presence of serum. In contrast, cells expanded on human amniotic membrane (AM) stromal matrix maintained a three-dimensional dendritic morphology and expressed keratocan mRNA and protein for at least 8 passages before senescence. When keratocytes were cultured on AM, the promoter activity of transforming growth factor (TGF)-beta2 and TGF-beta receptor II was down-regulated as compared with that on plastic. Furthermore, cells on AM continuously retained Smad 2 and Smad 4 in the cytoplasm and did not express alpha-smooth muscle actin, even when 10 ng/ml TGF-beta1 was added in a serum-free medium for up to 5 days. In parallel to such down-regulation of TGF-beta signaling, keratocan promoter-driven ECFP expression was observed in cells cultured either on AM in the presence of serum or on plastic containing serum treated with a neutralizing antibody to TGF-beta. Collectively, these results indicate that down-regulation of Smad-mediated TGF-beta signaling is an important mechanism for cultured keratocytes to maintain a normal phenotype while continuously expanded in a serum-containing medium. This strategy of suppressing TGF-beta signaling, achieved by AM stromal matrix in part via suppression of TGF-beta gene transcription, can be used to expand keratocytes in culture without the use of AM in the future.
Figures





References
-
- Beals MP, Funderburgh JL, Jester JV, Hassell JR. Invest Ophthalmol Vis Sci. 1999;40:1658–1663. - PubMed
-
- Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Cornea. 1996;15:505–516. - PubMed
-
- Espana EM, He H, Kawakita T, Di Pascuale MA, Raju VK, Liu CY, Tseng SC. Invest Ophthalmol Vis Sci. 2003;44:5136–5141. - PubMed
-
- Espana EM, Kawakita T, Liu CY, Tseng SCG. Invest Ophthalmol Vis Sci. 2004;45:2985–2991. - PubMed
-
- Dahl IM, Johnsen W, Anseth A, Prydz H. Exp Cell Res. 1974;88:193–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

