A critical role of T cell antigen receptor-transduced MHC class I-restricted helper T cells in tumor protection
- PMID: 15908507
- PMCID: PMC1142362
- DOI: 10.1073/pnas.0500357102
A critical role of T cell antigen receptor-transduced MHC class I-restricted helper T cells in tumor protection
Abstract
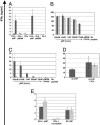
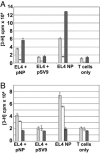
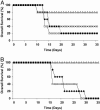
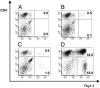
Adoptive transfer of antigen-specific CD4(+) and CD8(+) T cells is one of the most efficient forms of cancer immunotherapy. However, the isolation of antigen-specific CD4(+) T cells is limited because only few tumor-associated helper epitopes are identified. Here, we used T cell antigen receptor gene transfer to target CD4(+) T cells against an MHC class I-presented epitope of a model tumor antigen. IFN-gamma-producing CD4(+) T cells were unable to expand in vivo and to provide help for tumor rejection. In contrast, CD4(+) T cells producing high levels of IL-2 expanded in vivo, provided help for cytotoxic T lymphocyte-mediated tumor rejection, and developed T cell memory. The data demonstrate in vivo synergy between T cell antigen receptor-transduced CD4(+) and CD8(+) T cells specific for the same epitope resulting in long-term tumor protection.
Figures


Similar articles
-
Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor.Cancer Res. 2005 Feb 15;65(4):1570-6. doi: 10.1158/0008-5472.CAN-04-2076. Cancer Res. 2005. PMID: 15735047
-
Redirecting human CD4+ T lymphocytes to the MHC class I-restricted melanoma antigen MAGE-A1 by TCR alphabeta gene transfer requires CD8alpha.Gene Ther. 2005 Jan;12(2):140-6. doi: 10.1038/sj.gt.3302388. Gene Ther. 2005. PMID: 15496961
-
Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene.Mol Ther. 2006 Feb;13(2):310-9. doi: 10.1016/j.ymthe.2005.08.025. Epub 2005 Nov 4. Mol Ther. 2006. PMID: 16275163
-
MHC class II-restricted tumor antigens recognized by CD4+ T cells: new strategies for cancer vaccine design.J Immunother. 2001 May-Jun;24(3):195-204. J Immunother. 2001. PMID: 11394496 Review.
-
Genetic approaches for the induction of a CD4+ T cell response in cancer immunotherapy.J Gene Med. 2005 Jun;7(6):686-95. doi: 10.1002/jgm.713. J Gene Med. 2005. PMID: 15693037 Review.
Cited by
-
T cell receptor gene therapy for cancer.Hum Gene Ther. 2009 Nov;20(11):1240-8. doi: 10.1089/hum.2009.146. Hum Gene Ther. 2009. PMID: 19702439 Free PMC article. Review.
-
Transgenic CD8αβ co-receptor rescues endogenous TCR function in TCR-transgenic virus-specific T cells.J Immunother Cancer. 2020 Nov;8(2):e001487. doi: 10.1136/jitc-2020-001487. J Immunother Cancer. 2020. PMID: 33148692 Free PMC article.
-
TCR gene therapy of spontaneous prostate carcinoma requires in vivo T cell activation.J Immunol. 2008 Aug 15;181(4):2563-71. doi: 10.4049/jimmunol.181.4.2563. J Immunol. 2008. PMID: 18684947 Free PMC article.
-
TCR gene transfer: MAGE-C2/HLA-A2 and MAGE-A3/HLA-DP4 epitopes as melanoma-specific immune targets.Clin Dev Immunol. 2012;2012:586314. doi: 10.1155/2012/586314. Epub 2012 Feb 12. Clin Dev Immunol. 2012. PMID: 22400038 Free PMC article.
-
Changing viral tropism using immunoliposomes alters the stability of gene expression: implications for viral vector design.Mol Med. 2007 Mar-Apr;13(3-4):216-26. doi: 10.2119/2006–00052.Tan. Mol Med. 2007. PMID: 17592557 Free PMC article.
References
-
- Rooney, C. M., Smith, C. A., Ng, C. Y., Loftin, S., Li, C., Krance, R. A., Brenner, M. K. & Heslop, H. E. (1995) Lancet 345, 9–13. - PubMed
-
- Rooney, C. M., Smith, C. A., Ng, C. Y., Loftin, S. K., Sixbey, J. W., Gan, Y., Srivastava, D. K., Bowman, L. C., Krance, R. A., Brenner, M. K. & Heslop, H. E. (1998) Blood 92, 1549–1555. - PubMed
-
- Haque, T., Taylor, C., Wilkie, G. M., Murad, P., Amlot, P. L., Beath, S., McKiernan, P. J. & Crawford, D. H. (2001) Transplantation 72, 1399–1402. - PubMed
-
- Haque, T., Wilkie, G. M., Taylor, C., Amlot, P. L., Murad, P., Iley, A., Dombagoda, D., Britton, K. M., Swerdlow, A. J. & Crawford, D. H. (2002) Lancet 360, 436–442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

