KvAP-based model of the pore region of shaker potassium channel is consistent with cadmium- and ligand-binding experiments
- PMID: 15908577
- PMCID: PMC1366588
- DOI: 10.1529/biophysj.105.062240
KvAP-based model of the pore region of shaker potassium channel is consistent with cadmium- and ligand-binding experiments
Abstract
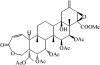
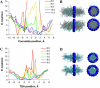
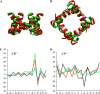
Potassium channels play fundamental roles in excitable cells. X-ray structures of bacterial potassium channels show that the pore-lining inner helices obstruct the cytoplasmic entrance to the closed channel KcsA, but diverge in widely open channels MthK and KvAP, suggesting a gating-hinge role for a conserved Gly in the inner helix. A different location of the gating hinge and a narrower open pore were proposed for voltage-gated Shaker potassium channels that have the Pro-473-Val-Pro motif. Two major observations back the proposal: cadmium ions lock mutant Val-476-Cys in the open state by bridging Cys-476 and His-486 in adjacent helices, and cadmium blocks the locked-open double mutant Val-474-Cys/Val-476-Cys by binding to Cys-474 residues. Here we used molecular modeling to show that the open Shaker should be as wide as KvAP to accommodate an open-channel blocker, correolide. We further built KvAP-, MthK-, and KcsA-based models of the Shaker mutants and Monte-Carlo-minimized them with constraints Cys-476-Cd(2+)-His-486. The latter were consistent with the KvAP-based model, causing a small-bend N-terminal to the Pro-473-Val-Pro motif. The constraints significantly distorted the MthK-based structure, making it similar to KvAP. The KcsA structure resisted the constraints. Two Cd(2+) ions easily block the locked-open KvAP-based model at Cys-474 residues, whereas constraining a single cadmium ion to four Cys-474 caused large conformational changes and electrostatic imbalance. Although mutual disposition of the voltage-sensor and pore domains in the KvAP x-ray structure is currently disputed, our results suggest that the pore-region domain retains a nativelike conformation in the crystal.
Figures

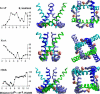

References
-
- Doyle, D. A., J. Morais Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. - PubMed
-
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B. T. Chait, and R. MacKinnon. 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. - PubMed
-
- Jiang, Y., A. Lee, J. Chen, V. Ruta, M. Cadene, B. T. Chait, and R. MacKinnon. 2003. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. - PubMed
-
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B. T. Chait, and R. MacKinnon. 2002. The open pore conformation of potassium channels. Nature. 417:523–526. - PubMed
-
- Luzhkov, V. B., J. Nilsson, P. Arhem, and J. Aqvist. 2003. Computational modelling of the open-state Kv 1.5 ion channel block by bupivacaine. Biochim. Biophys. Acta. 1652:35–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

