Transbilayer peptide sorting between raft and nonraft bilayers: comparisons of detergent extraction and confocal microscopy
- PMID: 15908585
- PMCID: PMC1366595
- DOI: 10.1529/biophysj.105.062380
Transbilayer peptide sorting between raft and nonraft bilayers: comparisons of detergent extraction and confocal microscopy
Abstract
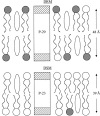
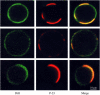
Membrane microdomains ("rafts") that sequester specific proteins and lipids are often characterized by their resistance to detergent extraction. Because rafts are enriched in sphingomyelin and cholesterol, raft bilayers are thicker and have larger area compressibility moduli than nonraft bilayers. It has been postulated that rafts concentrate proteins with long transmembrane domains (TMDs) because of "hydrophobic matching" between the TMDs and the thick raft bilayers. However, previous detergent extraction experiments with bilayers containing raft and nonraft domains have shown that the peptides P-23 and P-29, designed to have single TMDs matching the hydrocarbon thicknesses of detergent soluble membranes and detergent resistant membranes, respectively, are both localized to detergent soluble membranes. Those results imply that both peptides are preferentially located in nonraft domains. However, because the detergent solubilizes part of the bilayer, it has been unclear whether or not detergent extraction experiments provide an accurate indication of the location of peptides in intact bilayers. Here we use confocal microscopy to examine the distribution of these same peptides in intact bilayers containing both raft and nonraft domains. At 20 degrees C and 37 degrees C, P-23 and P-29 were both primarily localized in fluorescently labeled nonraft domains. These confocal results validate the previous detergent extraction experiments and demonstrate the importance of bilayer cohesive properties, compared to hydrophobic mismatch, in the sorting of these peptides that contain a single TMD.
Figures


Similar articles
-
Sorting of lens aquaporins and connexins into raft and nonraft bilayers: role of protein homo-oligomerization.Biophys J. 2009 Nov 4;97(9):2493-502. doi: 10.1016/j.bpj.2009.08.026. Biophys J. 2009. PMID: 19883592 Free PMC article.
-
Sorting of lipids and transmembrane peptides between detergent-soluble bilayers and detergent-resistant rafts.Biophys J. 2003 Sep;85(3):1656-66. doi: 10.1016/S0006-3495(03)74595-0. Biophys J. 2003. PMID: 12944280 Free PMC article.
-
X-ray diffraction to determine the thickness of raft and nonraft bilayers.Methods Mol Biol. 2007;398:221-30. doi: 10.1007/978-1-59745-513-8_15. Methods Mol Biol. 2007. PMID: 18214383
-
The state of lipid rafts: from model membranes to cells.Annu Rev Biophys Biomol Struct. 2003;32:257-83. doi: 10.1146/annurev.biophys.32.110601.142439. Epub 2003 Jan 16. Annu Rev Biophys Biomol Struct. 2003. PMID: 12543707 Review.
-
Lipid raft proteomics: more than just detergent-resistant membranes.Subcell Biochem. 2007;43:35-47. doi: 10.1007/978-1-4020-5943-8_4. Subcell Biochem. 2007. PMID: 17953390 Review.
Cited by
-
Membrane-mediated regulation of the intrinsically disordered CD3ϵ cytoplasmic tail of the TCR.Biophys J. 2015 May 19;108(10):2481-2491. doi: 10.1016/j.bpj.2015.03.059. Biophys J. 2015. PMID: 25992726 Free PMC article.
-
Structure elucidation of dimeric transmembrane domains of bitopic proteins.Cell Adh Migr. 2010 Apr-Jun;4(2):284-98. doi: 10.4161/cam.4.2.11930. Epub 2010 May 1. Cell Adh Migr. 2010. PMID: 20421711 Free PMC article. Review.
-
Sorting of lens aquaporins and connexins into raft and nonraft bilayers: role of protein homo-oligomerization.Biophys J. 2009 Nov 4;97(9):2493-502. doi: 10.1016/j.bpj.2009.08.026. Biophys J. 2009. PMID: 19883592 Free PMC article.
-
Lipid packing drives the segregation of transmembrane helices into disordered lipid domains in model membranes.Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1343-8. doi: 10.1073/pnas.1009362108. Epub 2011 Jan 4. Proc Natl Acad Sci U S A. 2011. PMID: 21205902 Free PMC article.
-
Orientation and dynamics of transmembrane peptides: the power of simple models.Eur Biophys J. 2010 Mar;39(4):609-21. doi: 10.1007/s00249-009-0567-1. Epub 2009 Dec 18. Eur Biophys J. 2010. PMID: 20020122 Free PMC article. Review.
References
-
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. - PubMed
-
- Field, K. A., D. Holowka, and B. Baird. 1997. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J. Biol. Chem. 272:4276–4280. - PubMed
-
- Baird, B., E. D. Sheets, and D. Holowka. 1999. How does the plasma membrane participate in cellular signaling by receptors for immunoglobulin E? Biophys. Chem. 82:109–119. - PubMed
-
- Kawabuchi, M., Y. Satomi, T. Takao, Y. Shimonishi, S. Nada, K. Nagai, A. Tarakhovsky, and M. Okada. 2000. Transmembrane phosphoprotein Chp regulates the activity of Src-family of tyrosine kinase. Nature. 404:999–1003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

