A molecular-genetic study of the Arabidopsis Toc75 gene family
- PMID: 15908591
- PMCID: PMC1150391
- DOI: 10.1104/pp.105.063289
A molecular-genetic study of the Arabidopsis Toc75 gene family
Abstract
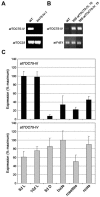
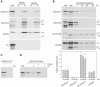
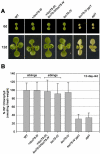
Toc75 (translocon at the outer envelope membrane of chloroplasts, 75 kD) is the protein translocation channel at the outer envelope membrane of plastids and was first identified in pea (Pisum sativum) using biochemical approaches. The Arabidopsis (Arabidopsis thaliana) genome contains three Toc75-related sequences, termed atTOC75-I, atTOC75-III, and atTOC75-IV, which we studied using a range of molecular, genetic, and biochemical techniques. Expression of atTOC75-III is strongly regulated and at its highest level in young, rapidly expanding tissues. By contrast, atTOC75-IV is expressed uniformly throughout development and at a much lower level than atTOC75-III. The third sequence, atTOC75-I, is a pseudogene that is not expressed due to a gypsy/Ty3 transposon insertion in exon 1, and numerous nonsense, frame-shift, and splice-junction mutations. The expressed genes, atTOC75-III and atTOC75-IV, both encode integral envelope membrane proteins. Unlike atToc75-III, the smaller atToc75-IV protein is not processed upon targeting to the envelope, and its insertion does not require ATP at high concentrations. The atTOC75-III gene is essential for viability, since homozygous atToc75-III knockout mutants (termed toc75-III) could not be identified, and aborted seeds were observed at a frequency of approximately 25% in the siliques of self-pollinated toc75-III heterozygotes. Homozygous toc75-III embryos were found to abort at the two-cell stage. Homozygous atToc75-IV knockout plants (termed toc75-IV) displayed no obvious visible phenotypes. However, structural abnormalities were observed in the etioplasts of toc75-IV seedlings and atTOC75-IV overexpressing lines, and toc75-IV plants were less efficient at deetiolation than wild type. These results suggest some role for atToc75-IV during growth in the dark.
Figures






References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Aronsson H, Jarvis P (2002) A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Lett 529: 215–220 - PubMed
-
- Bauer J, Chen K, Hiltbunner A, Wehrli E, Eugster M, Schnell D, Kessler F (2000) The major protein import receptor of plastids is essential for chloroplast biogenesis. Nature 403: 203–207 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

