Two homologous ATP-binding cassette transporter proteins, AtMDR1 and AtPGP1, regulate Arabidopsis photomorphogenesis and root development by mediating polar auxin transport
- PMID: 15908594
- PMCID: PMC1150410
- DOI: 10.1104/pp.105.061572
Two homologous ATP-binding cassette transporter proteins, AtMDR1 and AtPGP1, regulate Arabidopsis photomorphogenesis and root development by mediating polar auxin transport
Abstract
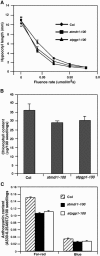
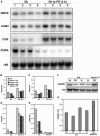
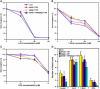
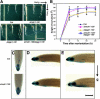
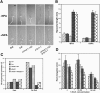
Light and auxin control many aspects of plant growth and development in an overlapping manner. We report here functional characterization of two closely related ABC (ATP-binding cassette) transporter genes, AtMDR1 and AtPGP1, in light and auxin responses. We showed that loss-of-function atmdr1 and atpgp1 mutants display hypersensitivity to far-red, red, and blue-light inhibition of hypocotyl elongation, reduced chlorophyll and anthocyanin accumulation, and abnormal expression of several light-responsive genes, including CAB3, RBCS, CHS, and PORA, under both darkness and far-red light conditions. In addition, we showed that the atmdr1-100 and atmdr1-100/atpgp1-100 mutants are defective in multiple aspects of root development, including increased root-growth sensitivity to 1-naphthalene acetic acid (1-NAA), and decreased sensitivity to naphthylphthalamic acid (NPA)-mediated inhibition of root elongation. Consistent with the proposed role of AtMDR1 in basipetal auxin transport, we found that expression of the auxin responsive DR5::GUS reporter gene in the central elongation zone is significantly reduced in the atmdr1-100 mutant roots treated with 1-NAA at the root tips, compared to similarly treated wild-type plants. Moreover, atmdr1-100, atpgp1-100, and their double mutants produced fewer lateral roots, in the presence or absence of 1-NAA or NPA. The atmdr1-100 and atmdr1-100/atpgp1-100 mutants also displayed enhanced root gravitropism. Genetic-epistasis analysis revealed that mutations in phyA largely suppress the randomized-hypocotyl growth and the short-hypocotyl phenotype of the atmdr1-100 mutants under far-red light, suggesting that phyA acts downstream of AtMDR1. Together, our results suggest that AtMDR1 and AtPGP1 regulate Arabidopsis (Arabidopsis thaliana) photomorphogenesis and multiple aspects of root development by mediating polar auxin transport.
Figures


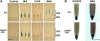

Similar articles
-
Characterization of a dwarf mutant allele of Arabidopsis MDR-like ABC transporter AtPGP1 gene.Biochem Biophys Res Commun. 2013 Nov 29;441(4):782-6. doi: 10.1016/j.bbrc.2013.10.136. Epub 2013 Nov 5. Biochem Biophys Res Commun. 2013. PMID: 24211579
-
TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19.Mol Biol Cell. 2003 Oct;14(10):4238-49. doi: 10.1091/mbc.e02-10-0698. Epub 2003 Aug 7. Mol Biol Cell. 2003. PMID: 14517332 Free PMC article.
-
Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis.Plant Physiol. 1998 Feb;116(2):455-62. doi: 10.1104/pp.116.2.455. Plant Physiol. 1998. PMID: 9489005 Free PMC article.
-
The ABC of auxin transport: the role of p-glycoproteins in plant development.FEBS Lett. 2006 Feb 13;580(4):1094-102. doi: 10.1016/j.febslet.2005.11.054. Epub 2005 Dec 6. FEBS Lett. 2006. PMID: 16359667 Review.
-
Auxin and Root Gravitropism: Addressing Basic Cellular Processes by Exploiting a Defined Growth Response.Int J Mol Sci. 2021 Mar 9;22(5):2749. doi: 10.3390/ijms22052749. Int J Mol Sci. 2021. PMID: 33803128 Free PMC article. Review.
Cited by
-
Connective auxin transport contributes to strigolactone-mediated shoot branching control independent of the transcription factor BRC1.PLoS Genet. 2019 Mar 13;15(3):e1008023. doi: 10.1371/journal.pgen.1008023. eCollection 2019 Mar. PLoS Genet. 2019. PMID: 30865619 Free PMC article.
-
Genome-wide identification of KANADI1 target genes.PLoS One. 2013 Oct 14;8(10):e77341. doi: 10.1371/journal.pone.0077341. eCollection 2013. PLoS One. 2013. PMID: 24155946 Free PMC article.
-
Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana.PLoS One. 2008 Jan 30;3(1):e1510. doi: 10.1371/journal.pone.0001510. PLoS One. 2008. PMID: 18231596 Free PMC article.
-
AtMRP6/AtABCC6, an ATP-binding cassette transporter gene expressed during early steps of seedling development and up-regulated by cadmium in Arabidopsis thaliana.BMC Plant Biol. 2008 Feb 28;8:22. doi: 10.1186/1471-2229-8-22. BMC Plant Biol. 2008. PMID: 18307782 Free PMC article.
-
Multiple roles for membrane-associated protein trafficking and signaling in gravitropism.Front Plant Sci. 2012 Dec 11;3:274. doi: 10.3389/fpls.2012.00274. eCollection 2012. Front Plant Sci. 2012. PMID: 23248632 Free PMC article.
References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Behringer FJ, Davies PJ (1992) Indole-3-acetic acid levels after phytochrome-mediated changes in the stem elongation rate of dark- and light-grown Pisum seedlings. Planta 188: 85–92 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

