ATP-sensitive K+ channel channel/enzyme multimer: metabolic gating in the heart
- PMID: 15910874
- PMCID: PMC2736952
- DOI: 10.1016/j.yjmcc.2005.02.022
ATP-sensitive K+ channel channel/enzyme multimer: metabolic gating in the heart
Abstract
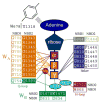
Cardiac ATP-sensitive K(+) (K(ATP)) channels, gated by cellular metabolism, are formed by association of the inwardly rectifying potassium channel Kir6.2, the potassium conducting subunit, and SUR2A, the ATP-binding cassette protein that serves as the regulatory subunit. Kir6.2 is the principal site of ATP-induced channel inhibition, while SUR2A regulates K(+) flux through adenine nucleotide binding and catalysis. The ATPase-driven conformations within the regulatory SUR2A subunit of the K(ATP) channel complex have determinate linkage with the states of the channel's pore. The probability and life-time of ATPase-induced SUR2A intermediates, rather than competitive nucleotide binding alone, defines nucleotide-dependent K(ATP) channel gating. Cooperative interaction, instead of independent contribution of individual nucleotide binding domains within the SUR2A subunit, serves a decisive role in defining K(ATP) channel behavior. Integration of K(ATP) channels with the cellular energetic network renders these channel/enzyme heteromultimers high-fidelity metabolic sensors. This vital function is facilitated through phosphotransfer enzyme-mediated transmission of controllable energetic signals. By virtue of coupling with cellular energetic networks and the ability to decode metabolic signals, K(ATP) channels set membrane excitability to match demand for homeostatic maintenance. This new paradigm in the operation of an ion channel multimer is essential in providing the basis for K(ATP) channel function in the cardiac cell, and for understanding genetic defects associated with life-threatening diseases that result from the inability of the channel complex to optimally fulfill its physiological role.
Figures

References
-
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. - PubMed
-
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, 4th, Boyd AE, 3rd, Gonzalez G, et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–6. - PubMed
-
- Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzales G, et al. Reconstitution of I KATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–70. - PubMed
-
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–76. - PubMed
-
- Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K_ channels. Neuron. 1996;16:1011–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

