Neurotrophin releasing single and multiple lumen nerve conduits
- PMID: 15911044
- PMCID: PMC2648409
- DOI: 10.1016/j.jconrel.2005.02.022
Neurotrophin releasing single and multiple lumen nerve conduits
Abstract
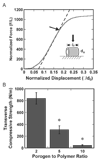
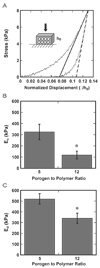
Tissue engineering strategies for nerve repair employ polymer conduits termed guidance channels and bridges to promote regeneration for peripheral nerve injury and spinal cord injury, respectively. An approach for fabrication of nerve conduits with single and multiple lumens capable of controlled release of neurotrophic factors was developed. These conduits were fabricated from a mixture of poly(lactide-co-glycolide) (PLG) microspheres and porogen (NaCl) that was loaded into a mold and processed by gas foaming. The porosity and mechanical properties of the constructs were regulated by the ratio of porogen to polymer microsphere. The neurotrophin, nerve growth factor (NGF), was incorporated into the conduit by either mixing the protein with microspheres or encapsulating the protein within microspheres prior to gas foaming. A sustained release was observed for at least 42 days, with the release rate controlled by method of incorporation and polymer molecular weight. Released NGF retained its bioactivity, as demonstrated by its ability to stimulate neurite outgrowth from primary dorsal root ganglion (DRG). In vivo results indicate that conduits retain their original architecture, and allow for cellular infiltration into the channels. Polymer conduits with controllable lumen diameters and protein release may enhance nerve regeneration by guiding and stimulating neurite outgrowth.
Figures






Similar articles
-
Novel drug delivering conduit for peripheral nerve regeneration.J Neural Eng. 2017 Dec;14(6):066011. doi: 10.1088/1741-2552/aa867d. J Neural Eng. 2017. PMID: 28829045
-
Nerve growth factor expression by PLG-mediated lipofection.Biomaterials. 2006 Apr;27(11):2477-86. doi: 10.1016/j.biomaterials.2005.11.016. Epub 2005 Nov 28. Biomaterials. 2006. PMID: 16316681 Free PMC article.
-
Polyphosphoester microspheres for sustained release of biologically active nerve growth factor.Biomaterials. 2002 Sep;23(17):3765-72. doi: 10.1016/s0142-9612(02)00116-3. Biomaterials. 2002. PMID: 12109702
-
Combining growth factor releasing microspheres within aligned nanofibers enhances neurite outgrowth.J Biomed Mater Res A. 2018 Jan;106(1):17-25. doi: 10.1002/jbm.a.36204. Epub 2017 Sep 23. J Biomed Mater Res A. 2018. PMID: 28879680
-
Controlling the dose-dependent, synergistic and temporal effects of NGF and GDNF by encapsulation in PLGA microparticles for use in nerve guidance conduits for the repair of large peripheral nerve defects.J Control Release. 2019 Jun 28;304:51-64. doi: 10.1016/j.jconrel.2019.05.001. Epub 2019 May 2. J Control Release. 2019. PMID: 31054993
Cited by
-
Patterned PLG substrates for localized DNA delivery and directed neurite extension.Biomaterials. 2007 Jun;28(16):2603-11. doi: 10.1016/j.biomaterials.2007.01.042. Epub 2007 Feb 9. Biomaterials. 2007. PMID: 17324456 Free PMC article.
-
Biomimetic micropatterned multi-channel nerve guides by templated electrospinning.Biotechnol Bioeng. 2012 Jun;109(6):1571-82. doi: 10.1002/bit.24412. Epub 2012 Jan 2. Biotechnol Bioeng. 2012. PMID: 22179932 Free PMC article.
-
Biomaterial Approaches to Modulate Reactive Astroglial Response.Cells Tissues Organs. 2018;205(5-6):372-395. doi: 10.1159/000494667. Epub 2018 Dec 5. Cells Tissues Organs. 2018. PMID: 30517922 Free PMC article. Review.
-
From molecular to nanotechnology strategies for delivery of neurotrophins: emphasis on brain-derived neurotrophic factor (BDNF).Pharmaceutics. 2013 Feb 8;5(1):127-67. doi: 10.3390/pharmaceutics5010127. Pharmaceutics. 2013. PMID: 24300402 Free PMC article.
-
Bridging the lesion-engineering a permissive substrate for nerve regeneration.Regen Biomater. 2015 Sep;2(3):203-14. doi: 10.1093/rb/rbv012. Epub 2015 Aug 10. Regen Biomater. 2015. PMID: 26816642 Free PMC article.
References
-
- Evans GR. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat. Rec. 2001;263(4):396–404. - PubMed
-
- Geller HM, Fawcett JW. Building a bridge: engineering spinal cord repair. Exp. Neurol. 2002;174(2):125–136. - PubMed
-
- Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 2003;5:293–347. - PubMed
-
- Talac R, et al. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials. 2004;25(9):1505–1510. - PubMed
-
- Aebischer P, Salessiotis AN, Winn SR. Basic fibroblast growth factor released from synthetic guidance channels facilitates peripheral nerve regeneration across long nerve gaps. J. Neurosci. Res. 1989;23(3):282–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

