Composite genome map and recombination parameters derived from three archetypal lineages of Toxoplasma gondii
- PMID: 15911631
- PMCID: PMC1137028
- DOI: 10.1093/nar/gki604
Composite genome map and recombination parameters derived from three archetypal lineages of Toxoplasma gondii
Abstract
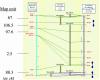
Toxoplasma gondii is a highly successful protozoan parasite in the phylum Apicomplexa, which contains numerous animal and human pathogens. T.gondii is amenable to cellular, biochemical, molecular and genetic studies, making it a model for the biology of this important group of parasites. To facilitate forward genetic analysis, we have developed a high-resolution genetic linkage map for T.gondii. The genetic map was used to assemble the scaffolds from a 10X shotgun whole genome sequence, thus defining 14 chromosomes with markers spaced at approximately 300 kb intervals across the genome. Fourteen chromosomes were identified comprising a total genetic size of approximately 592 cM and an average map unit of approximately 104 kb/cM. Analysis of the genetic parameters in T.gondii revealed a high frequency of closely adjacent, apparent double crossover events that may represent gene conversions. In addition, we detected large regions of genetic homogeneity among the archetypal clonal lineages, reflecting the relatively few genetic outbreeding events that have occurred since their recent origin. Despite these unusual features, linkage analysis proved to be effective in mapping the loci determining several drug resistances. The resulting genome map provides a framework for analysis of complex traits such as virulence and transmission, and for comparative population genetic studies.
Figures



References
-
- Joynson D.H.M., Wreghitt T.G. Toxoplasmosis: A Comprehensive Clinical Guide. Cambridge: Cambridge University Press; 2001. p. 395.
-
- Dubey J.P. In: Parasitic Protozoa. Kreier J.P., editor. New York: Academic Press; 1977. pp. 101–237.
-
- Roos D.S., Donald R.G.K., Morrissette N.S., Moulton A.L. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. - PubMed

