Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments
- PMID: 15911768
- PMCID: PMC1138261
- DOI: 10.1073/pnas.0502206102
Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments
Abstract
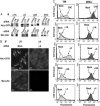
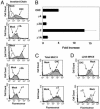
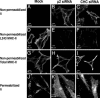
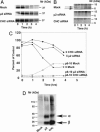
Major histocompatibility complex class II (MHC-II) molecules are composed of two polymorphic chains, alpha and beta, which assemble with an invariant chain, Ii, in the endoplasmic reticulum. The assembled MHC-II complexes are transported to the Golgi complex and then to late endosomes/lysosomes, where Ii is degraded and alphabeta dimers bind peptides derived from exogenous antigens. Targeting of MHC-II molecules to these compartments is mediated by two dileucine-based signals in the cytoplasmic domain of Ii. These signals bind in vitro to two adaptor protein (AP) complexes, AP-1 and AP-2, which are components of clathrin coats involved in vesicle formation and cargo sorting. The physiological roles of these proteins in MHC-II molecule trafficking, however, remain to be addressed. Here, we report the use of RNA interference to examine the involvement of clathrin and four AP complexes (AP-1, AP-2, AP-3, and AP-4) in MHC-II molecule trafficking in vivo. We found that depletion of clathrin or AP-2 caused >10-fold increases in Ii expression on the cell surface and a concomitant decrease in Ii localization to endosomal/lysosomal vesicles. In addition, depletion of clathrin or AP-2 delayed the degradation of Ii and reduced the surface expression of peptide-loaded alphabeta dimers. In contrast, depletion of AP-1, AP-3, or AP-4 had little or no effect. These findings demonstrate that clathrin and AP-2 participate in MHC-II molecule trafficking in vivo. Because AP-2 is only associated with the plasma membrane, these results also indicate that a significant pool of MHC-II molecules traffic to the endosomal-lysosomal system by means of the cell surface.
Figures

References
-
- Holling, T. M., Schooten, E. & Van Den Elsen, P. J. (2004) Hum. Immunol. 65, 282–290. - PubMed
-
- Peters, P. J., Neefjes, J. J., Oorschot, V., Ploegh, H. L. & Geuze, H. J. (1991) Nature 349, 669–676. - PubMed
-
- Castellino, F. & Germain, R. N. (1995) Immunity 2, 73–88. - PubMed
-
- Cresswell, P. (1994) Annu. Rev. Immunol. 12, 259–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

