A sensitive flow cytometric methodology for studying the binding of L. chagasi to canine peritoneal macrophages
- PMID: 15913461
- PMCID: PMC1166554
- DOI: 10.1186/1471-2334-5-39
A sensitive flow cytometric methodology for studying the binding of L. chagasi to canine peritoneal macrophages
Abstract
Background: The Leishmania promastigote-macrophage interaction occurs through the association of multiple receptors on the biological membrane surfaces. The success of the parasite infection is dramatically dependent on this early interaction in the vertebrate host, which permits or not the development of the disease. In this study we propose a novel methodology using flow cytometry to study this interaction, and compare it with a previously described "in vitro" binding assay.
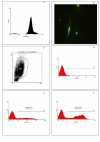
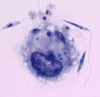
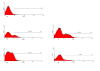
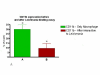
Methods: To study parasite-macrophage interaction, peritoneal macrophages were obtained from 4 dogs and adjusted to 3 x 10(6) cells/mL. Leishmania (Leishmania) chagasi parasites (stationary-phase) were adjusted to 5 x 10(7) cells/mL. The interaction between CFSE-stained Leishmania chagasi and canine peritoneal macrophages was performed in polypropylene tubes to avoid macrophage adhesion. We carried out assays in the presence or absence of normal serum or in the presence of a final concentration of 5% of C5 deficient (serum from AKR/J mice) mouse serum. Then, the number of infected macrophages was counted in an optical microscope, as well as by flow citometry. Macrophages obtained were stained with anti-CR3 (CD11b/CD18) antibodies and analyzed by flow citometry.
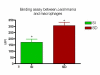
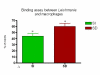
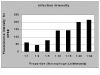
Results: Our results have shown that the interaction between Leishmania and macrophages can be measured by flow cytometry using the fluorescent dye CFSE to identify the Leishmania, and measuring simultaneously the expression of an important integrin involved in this interaction: the CD11b/CD18 (CR3 or Mac-1) beta2 integrin.
Conclusion: Flow cytometry offers rapid, reliable and sensitive measurements of single cell interactions with Leishmania in unstained or phenotypically defined cell populations following staining with one or more fluorochromes.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

