The C-terminal tail of the gp41 transmembrane envelope glycoprotein of HIV-1 clades A, B, C, and D may exist in two conformations: an analysis of sequence, structure, and function
- PMID: 15913700
- PMCID: PMC7111842
- DOI: 10.1016/j.virol.2005.04.015
The C-terminal tail of the gp41 transmembrane envelope glycoprotein of HIV-1 clades A, B, C, and D may exist in two conformations: an analysis of sequence, structure, and function
Abstract
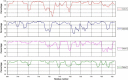
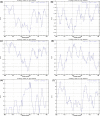
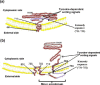
In addition to the major ectodomain, the gp41 transmembrane glycoprotein of HIV-1 is now known to have a minor ectodomain that is part of the long C-terminal tail. Both ectodomains are highly antigenic, carry neutralizing and non-neutralizing epitopes, and are involved in virus-mediated fusion activity. However, data have so far been biologically based, and derived solely from T cell line-adapted (TCLA), B clade viruses. Here we have carried out sequence and theoretically based structural analyses of 357 gp41 C-terminal sequences of mainly primary isolates of HIV-1 clades A, B, C, and D. Data show that all these viruses have the potential to form a tail loop structure (the minor ectodomain) supported by three, beta-sheet, membrane-spanning domains (MSDs). This means that the first (N-terminal) tyrosine-based sorting signal of the gp41 tail is situated outside the cell membrane and is non-functional, and that gp41 that reaches the cell surface may be recycled back into the cytoplasm through the activity of the second tyrosine-sorting signal. However, we suggest that only a minority of cell-associated gp41 molecules - those destined for incorporation into virions - has 3 MSDs and the minor ectodomain. Most intracellular gp41 has the conventional single MSD, no minor ectodomain, a functional first tyrosine-based sorting signal, and in line with current thinking is degraded intracellularly. The gp41 structural diversity suggested here can be viewed as an evolutionary strategy to minimize HIV-1 envelope glycoprotein expression on the cell surface, and hence possible cytotoxicity and immune attack on the infected cell.
Figures

References
-
- Berlioz-Torrent C., Shacklett B.L., Erdtmann L., Delamarre L., Bouchaert I., Sonigo P., Dokhelar M.C., Benarous R. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adapter complexes modulate intracellular and cell surface expression of envelope glycoprotein. J. Virol. 1999;73:1350–1361. - PMC - PubMed
-
- Bird C., Burke J., Gleeson P.A., McCluskey J. Expression of human immunodeficiency virus type 1 (HIV-1) envelope gene products transcribed from a heterologous promoter. J. Biol. Chem. 1990;265:19151–19157. - PubMed
-
- Boge M., Wyss S., Bonifacino J.S., Thali M. A membrane-proximal signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 1998;273:15773–15778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

