Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010
- PMID: 15914552
- PMCID: PMC1895225
- DOI: 10.1182/blood-2005-04-1437
Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010
Abstract
The addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy results in significant improvement in clinical outcome for individuals with non-HIV-associated aggressive B-cell lymphoma. To assess the potential risks and benefits of the addition of rituximab to CHOP for HIV-associated non-Hodgkin lymphoma (HIV-NHL) 150 patients receiving CHOP for HIV-NHL were randomized (2:1) to receive 375 mg/m(2) rituximab with each chemotherapy cycle (n = 99) or no immunotherapy (n = 50) in a multicenter phase 3 trial. The complete response rate (CR + CRu) was 57.6% for R-CHOP and 47% for CHOP (P = .147). With a median follow-up of 137 weeks, time to progression, progression-free survival, and overall survival times were 125, 45, and 139 weeks, respectively, for R-CHOP and 85, 38, and 110 weeks, respectively, for CHOP (P = not significant, all comparisons). Treatment-related infectious deaths occurred in 14% of patients receiving R-CHOP compared with 2% in the chemotherapy-alone group (P = .035). Of these deaths, 60% occurred in patients with CD4 counts less than 50/mm(3). Progression-free survival was significantly influenced by CD4(+) count (P < .001) and International Prognostic Index score (P = .022), but not bcl-2 status. The addition of rituximab to CHOP in patients with HIV-NHL may be associated with improved tumor responses. However, these benefits may be offset by an increase in infectious deaths, particularly in those individuals with CD4(+) lymphocyte counts less than 50/mm(3).
Figures

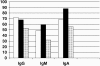
 ).
).Comment in
-
The infectious consequences of rituximab addition to fludarabine-containing regimens.Blood. 2006 Apr 1;107(7):3013-4. doi: 10.1182/blood-2005-10-4012. Blood. 2006. PMID: 16554491 No abstract available.
-
The case for rituximab in AIDS-related lymphoma.Blood. 2006 Apr 1;107(7):3014-5. doi: 10.1182/blood-2005-09-3885. Blood. 2006. PMID: 16554492 No abstract available.
References
-
- Gates AE, Kaplan LD. AIDS malignancies in the era of highly active antiretroviral therapy, part I. Oncology. 2002;16: 441-459. - PubMed
-
- Revision of the case definition of acquired immunodeficiency syndrome for national reporting—United States. MMWR Morb Mortal Wkly Rep. 1985;34: 373-375. - PubMed
-
- Appleby P, Beral V, Newton R, Reeves G. Highly active antiretroviral therapy and the incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92: 1823-1830. - PubMed
-
- Gates AE, Kaplan LD. Biology and management of AIDS-associated non-Hodgkin's lymphoma. Hematol Oncol Clin North Am. 2003;17: 821-841. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- U01C-A070 072/PHS HHS/United States
- U01CA070 054/CA/NCI NIH HHS/United States
- U01CA070 062/CA/NCI NIH HHS/United States
- U01CA70 058/CA/NCI NIH HHS/United States
- U01CA070 080/CA/NCI NIH HHS/United States
- U01CA070 019/CA/NCI NIH HHS/United States
- U01CA083 038/CA/NCI NIH HHS/United States
- U01CA083 035/CA/NCI NIH HHS/United States
- U01CA083 118/CA/NCI NIH HHS/United States
- U01CA071 375/CA/NCI NIH HHS/United States
- U01CA070 079/CA/NCI NIH HHS/United States
- U01CA070 047/CA/NCI NIH HHS/United States
- U01CA070 081/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

