A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids
- PMID: 15914665
- PMCID: PMC1140087
- DOI: 10.1093/nar/gni085
A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids
Abstract
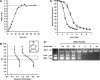
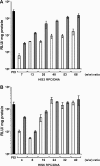
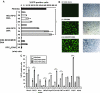
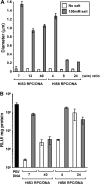
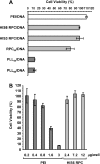
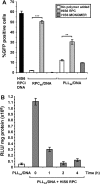
Synthetic vectors based on reducible polycations consisting of histidine and polylysine residues (HIS RPCs) were evaluated for their ability to deliver nucleic acids. Initial experiments showed that RPC-based vectors with at least 70% histidine content mediated efficient levels of gene transfer without requirement for the endosomolytic agent chloroquine. Significant gene transfer was observed in a range of cell types achieving up to a 5-fold increase in the percentage of transfected cells compared to 25 kDa PEI, a gold standard synthetic vector. In contrast to 25 kDa PEI, HIS RPCs also mediated efficient transfer of other nucleic acids, including mRNA encoding green fluorescent protein in PC-3 cells and siRNA directed against the neurotrophin receptor p75(NTR) in post-mitotic cultures of rat dorsal root ganglion cell neurons. Experiments to elevate intracellular glutathione and linear profiling of cell images captured by multiphoton fluorescent microscopy highlighted that parameters such as the molecular weight and rate of cleavage of HIS RPCs were important factors in determining transfection activity. Altogether, these results demonstrate that HIS RPCs represent a novel and versatile type of vector that can be used for efficient cytoplasmic delivery of a broad range of nucleic acids. This should enable different or a combination of therapeutic strategies to be evaluated using a single type of polycation-based vector.
Figures



References
-
- Dorsett Y., Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nature Rev. Drug. Discov. 2004;3:318–329. - PubMed
-
- Buckley R.H. Gene therapy for SCID—a complication after remarkable progress. Lancet. 2002;360:1185–1186. - PubMed
-
- European Society of Gene Therapy. French gene therapy group reports on the adverse event in a clinical trial of gene therapy for X-linked severe combined immune deficiency (X-SCID) J. Gene Med. 2003;5:82–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

