Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life
- PMID: 15916483
- PMCID: PMC3181105
- DOI: 10.1089/hum.2005.16.584
Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life
Abstract
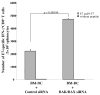
Dendritic cell-based vaccines have become an important approach for the treatment of malignancies. Numerous techniques have recently been designed to optimize dendritic cell activation, tumor antigen delivery to dendritic cells, and induction of tumor-specific immune responses in vivo. Dendritic cells (DCs), however, have a limited life span because they are subject to apoptotic cell death mediated by T cells, hindering their long-term ability to prime antigen-specific T cells. Small interfering RNA targeting Bak and Bax antiapoptotic proteins can be used to allow transfected DCs to resist killing by T cells in vivo. In this study, we show that human papillomavirus E7-loaded dendritic cells transfected with BAK/BAX siRNA downregulate Bak and Bax protein expression and become resistant to killing by T cells, leading to enhanced E7-specific CD8+ T cell activation and antitumor effects in vivo. More importantly, we found that vaccination with E7-loaded DCs transfected with BAK/BAX siRNA was capable of generating a strong therapeutic effect in vaccinated mice, compared with DCs transfected with control siRNA. Our data indicate that transfection of dendritic cells with BAK/BAX siRNA represents a plausible strategy for enhancing dendritic cell-based vaccine potency.
Figures


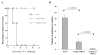


Similar articles
-
Enhancing dendritic cell vaccine potency by combining a BAK/BAX siRNA-mediated antiapoptotic strategy to prolong dendritic cell life with an intracellular strategy to target antigen to lysosomal compartments.Int J Cancer. 2007 Apr 15;120(8):1696-703. doi: 10.1002/ijc.22377. Int J Cancer. 2007. PMID: 17230516
-
Enhancement of dendritic cell-based vaccine potency by anti-apoptotic siRNAs targeting key pro-apoptotic proteins in cytotoxic CD8(+) T cell-mediated cell death.Immunol Lett. 2009 Jan 29;122(1):58-67. doi: 10.1016/j.imlet.2008.12.006. Epub 2009 Jan 9. Immunol Lett. 2009. PMID: 19135479
-
The siRNA cocktail targeting interleukin 10 receptor and transforming growth factor-β receptor on dendritic cells potentiates tumour antigen-specific CD8(+) T cell immunity.Clin Exp Immunol. 2015 Jul;181(1):164-78. doi: 10.1111/cei.12620. Epub 2015 May 17. Clin Exp Immunol. 2015. PMID: 25753156 Free PMC article.
-
New developments in dendritic cell-based vaccinations: RNA translated into clinics.Cancer Immunol Immunother. 2005 Jun;54(6):517-25. doi: 10.1007/s00262-004-0605-x. Epub 2005 Jan 27. Cancer Immunol Immunother. 2005. PMID: 15838706 Free PMC article. Review.
-
Antigen receptors and dendritic cells.Vaccine. 2000 Feb 25;18(16):1603-5. doi: 10.1016/s0264-410x(99)00493-4. Vaccine. 2000. PMID: 10689135 Review.
Cited by
-
Dendritic cells in cancer immunotherapy: vaccines or autologous transplants?Immunol Res. 2011 Aug;50(2-3):235-47. doi: 10.1007/s12026-011-8224-z. Immunol Res. 2011. PMID: 21717071 Free PMC article.
-
DNA vaccines delivered by human papillomavirus pseudovirions as a promising approach for generating antigen-specific CD8+ T cell immunity.Cell Biosci. 2011 Jul 28;1:26. doi: 10.1186/2045-3701-1-26. Cell Biosci. 2011. PMID: 21798027 Free PMC article.
-
Immunological research using RNA interference technology.Immunology. 2007 Jul;121(3):295-307. doi: 10.1111/j.1365-2567.2007.02599.x. Epub 2007 Apr 12. Immunology. 2007. PMID: 17428313 Free PMC article. Review.
-
Cervical Cancer: Development of Targeted Therapies Beyond Molecular Pathogenesis.Curr Obstet Gynecol Rep. 2014 Mar 1;3(1):18-32. doi: 10.1007/s13669-013-0068-1. Curr Obstet Gynecol Rep. 2014. PMID: 24533233 Free PMC article.
-
Antigen-specific immunotherapy of cervical and ovarian cancer.Immunol Rev. 2008 Apr;222:43-69. doi: 10.1111/j.1600-065X.2008.00622.x. Immunol Rev. 2008. PMID: 18363994 Free PMC article. Review.
References
-
- CERUNDOLO V, HERMANS IF, SALIO M. Dendritic cells: A journey from laboratory to clinic. Nat Immunol. 2004;5:7–10. - PubMed
-
- DEGLI ESPOSTI M, DIVE C. Mitochondrial membrane permeabilisation by Bax/Bak. Biochem Biophys Res Commun. 2003;304:455–461. - PubMed
-
- ENGLEMAN EG. Dendritic cell-based cancer immunotherapy. Semin Oncol. 2003;30:23–29. - PubMed
-
- FELTKAMP MC, SMITS HL, VIERBOOM MP, MINNAAR RP, DE JB, DRIJFHOUT JW, TER SJ, MELIEF CJ, KAST WM. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. - PubMed
-
- FIGDOR CG, DE VRIES IJ, LESTERHUIS WJ, MELIEF CJ. Dendritic cell immunotherapy: Mapping the way. Nat Med. 2004;10:475–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

