Trypanosoma cruzi posttranscriptionally up-regulates and exploits cellular FLIP for inhibition of death-inducing signal
- PMID: 15917295
- PMCID: PMC1182294
- DOI: 10.1091/mbc.e04-12-1051
Trypanosoma cruzi posttranscriptionally up-regulates and exploits cellular FLIP for inhibition of death-inducing signal
Abstract
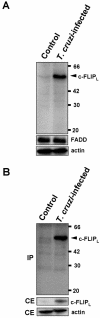
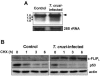
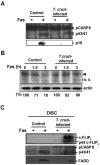
Intracellular persistence of the protozoan parasite, Trypanosoma cruzi, is an aggravating cause of Chagas' disease, involving that the protozoan infection specifically inhibits death receptor-mediated apoptosis of host cells. Here we demonstrate that the parasite dramatically up-regulates cellular FLICE inhibitory protein (c-FLIP), the only known mammalian inhibitor specific for death receptor signaling, in infected cells by an unusual, posttranscriptional stabilization of the short-lived protein. We also show that c-FLIP is accumulated in T. cruzi-infected mouse heart muscle cells in vivo. Stimulation of death receptor Fas in infected cells induces recruitment of c-FLIP to block the procaspase-8 activation at the most upstream caspase cascade. c-FLIP knock-down with a small interfering RNA significantly restores Fas-mediated apoptosis in infected cells. Taken together, our findings indicate that T. cruzi posttranscriptionally up-regulates and exploits host c-FLIP for the inhibition of death-inducing signal, a mechanism that may allow parasites to persist in host cells.
Figures


References
-
- Benedict, C. A., Norris, P. S., and Ware, C. F. (2002). To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3, 1013–1018. - PubMed
-
- Beverley, S. M. (1996). Hijacking the cell: parasites in the driver's seat. Cell 87, 787–789. - PubMed
-
- Brener, Z. (1973). Biology of Trypanosoma cruzi. Annu. Rev. Microbiol. 27, 347–382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

