Conditional induction of ovulation in mice
- PMID: 15917351
- PMCID: PMC1764799
- DOI: 10.1095/biolreprod.104.039164
Conditional induction of ovulation in mice
Abstract
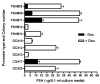
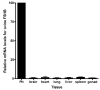
Follicle-stimulating hormone controls the maturation of mammalian ovarian follicles. In excess, it can increase ovulation (egg production). Reported here is a transgenic doxycycline-activated switch, tested in mice, that produced more FSHB subunit (therefore more FSH) and increased ovulation by the simple feeding of doxycycline (Dox). The transgenic switch was expressed selectively in pituitary gonadotropes and was designed to enhance normal expression of FSH when exposed to Dox, but to be regulated by all the hormones that normally control FSH production in vivo. Feeding maximally effective levels of Dox increased overall mRNA for FSHB and serum FSH by over half in males, and Dox treatment more than doubled the normal ovulation rate of female mice for up to 10 reproductive cycles. Lower levels of Dox increased the number of developing embryos by 30%. Ovarian structure and function appeared normal. In summary, gene switch technology and normal FSH regulation were combined to effectively enhance ovulation in mice. Theoretically, the same strategy can be used with any genetic switch to increase ovulation (or any highly conserved physiology) in any mammal.
Figures


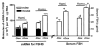

References
-
- Pursel VG, Bolt DJ, Miller KF, Pinkert CA, Hammer RE, Palmiter RD, Brinster RL. Expression and performance in transgenic pigs. J Reprod Fertil Suppl. 1990;40:235–245. - PubMed
-
- Kumar TR, Palapattu G, Wang P, Woodruff TK, Boime I, Byrne MC, Matzuk MM. Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol Endocrinol. 1999;13:851–865. - PubMed
-
- Zhu Z, Sheng T, Lee CG, Homer RJ, Elias JA. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin Cell Dev Bio. 2002;13:121–128. - PubMed
-
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. - PubMed
-
- Miller CD, Miller WL. Transcriptional repression of the ovine follicle-stimulating hormone-beta gene by 17 beta-estradiol. Endocrinology. 1996;137:3437–3446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

