Stimulation of the XPB ATP-dependent helicase by the beta subunit of TFIIE
- PMID: 15917439
- PMCID: PMC1140373
- DOI: 10.1093/nar/gki623
Stimulation of the XPB ATP-dependent helicase by the beta subunit of TFIIE
Abstract
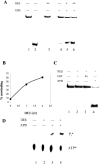
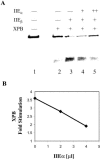
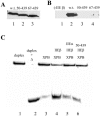
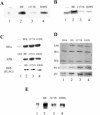
TFIIE and TFIIH are essential for the promoter opening and escape that occurs as RNA polymerase II transits into early elongation. XPB, a subunit of TFIIH, contains an ATP-dependent helicase activity that is used in both of these processes. Here, we show that the smaller beta subunit of TFIIE stimulates the XPB helicase and ATPase activities. The larger alpha subunit can use its known inhibitory activity to moderate the stimulation by the beta subunit. Regions of TFIIE beta required for the helicase stimulation were identified. Mutants were constructed that are defective in stimulating the XPB helicase but still allow intact TFIIE to bind and recruit XPB and TFIIH to form the pre-initiation complex. In a test for the functional significance of the stimulatory effect of TFIIE beta, these mutant forms of TFIIE were shown to be defective in a transcription assay on linear DNA. The data suggest that the beta subunit of TFIIE is an ATPase and helicase co-factor that can assist the XPB subunit of TFIIH during transcription initiation and the transition to early elongation, enhancing the potential diversity of regulatory targets.
Figures


Similar articles
-
A role for the TFIIH XPB DNA helicase in promoter escape by RNA polymerase II.J Biol Chem. 1999 Aug 6;274(32):22127-30. doi: 10.1074/jbc.274.32.22127. J Biol Chem. 1999. PMID: 10428772
-
Regulation of TFIIH ATPase and kinase activities by TFIIE during active initiation complex formation.Nature. 1994 Mar 10;368(6467):160-3. doi: 10.1038/368160a0. Nature. 1994. PMID: 8166891
-
Transcription without XPB Establishes a Unified Helicase-Independent Mechanism of Promoter Opening in Eukaryotic Gene Expression.Mol Cell. 2017 Feb 2;65(3):504-514.e4. doi: 10.1016/j.molcel.2017.01.012. Mol Cell. 2017. PMID: 28157507
-
[Structural biology of a general transcription factor, TFIIE and its interaction mode with TFIIH].Seikagaku. 2008 Jun;80(6):501-10. Seikagaku. 2008. PMID: 18634425 Review. Japanese. No abstract available.
-
XPB: An unconventional SF2 DNA helicase.Prog Biophys Mol Biol. 2015 Mar;117(2-3):174-181. doi: 10.1016/j.pbiomolbio.2014.12.005. Epub 2015 Jan 30. Prog Biophys Mol Biol. 2015. PMID: 25641424 Review.
Cited by
-
Subunit architecture of general transcription factor TFIIH.Proc Natl Acad Sci U S A. 2012 Feb 7;109(6):1949-54. doi: 10.1073/pnas.1105266109. Epub 2012 Jan 20. Proc Natl Acad Sci U S A. 2012. PMID: 22308316 Free PMC article.
-
Other proteins interacting with XP proteins.Adv Exp Med Biol. 2008;637:103-12. doi: 10.1007/978-0-387-09599-8_11. Adv Exp Med Biol. 2008. PMID: 19181115 Free PMC article. Review. No abstract available.
-
GTF2E2 Mutations Destabilize the General Transcription Factor Complex TFIIE in Individuals with DNA Repair-Proficient Trichothiodystrophy.Am J Hum Genet. 2016 Apr 7;98(4):627-42. doi: 10.1016/j.ajhg.2016.02.008. Epub 2016 Mar 17. Am J Hum Genet. 2016. PMID: 26996949 Free PMC article.
-
Trichothiodystrophy causative TFIIEβ mutation affects transcription in highly differentiated tissue.Hum Mol Genet. 2017 Dec 1;26(23):4689-4698. doi: 10.1093/hmg/ddx351. Hum Mol Genet. 2017. PMID: 28973399 Free PMC article.
-
Architecture of the RNA polymerase II preinitiation complex and mechanism of ATP-dependent promoter opening.Nat Struct Mol Biol. 2012 Aug;19(8):788-96. doi: 10.1038/nsmb.2334. Epub 2012 Jul 1. Nat Struct Mol Biol. 2012. PMID: 22751016 Free PMC article.
References
-
- Orphanides G., Lagrange T., Reinberg D. The general transcription factors of RNA polymerase II. Genes Dev. 1996;10:2657–2683. - PubMed
-
- Lee T.I., Young R.A. Transcription of eukaryotic protein-coding genes. Annu. Rev. Genet. 2000;34:77–137. - PubMed
-
- Buratowski S., Hahn S., Guarente L., Sharp P.A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell. 1989;56:549–561. - PubMed
-
- Dvir A., Conaway J.W., Conaway R.C. Mechanism of transcription initiation and promoter escape by RNA polymerase II. Curr. Opin. Genet. Dev. 2001;11:209–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

