Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling
- PMID: 15917451
- PMCID: PMC6724810
- DOI: 10.1523/JNEUROSCI.0237-05.2005
Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling
Abstract
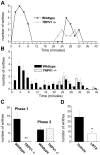
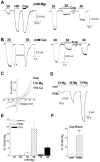
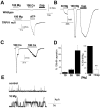
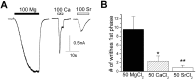
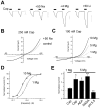
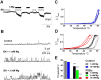
Transient receptor potential (TRP) channels detect diverse sensory stimuli, including alterations in osmolarity. However, a molecular detector of noxious hypertonic stimuli has not yet been identified. We show here that acute pain-related behavior evoked by elevated ionic strength is abolished in TRP vanilloid subtype 1 (TRPV1)-null mice and inhibited by iodoresiniferatoxin, a potent TRPV1 antagonist. Electrophysiological recordings demonstrate a novel form of ion channel modulation by which extracellular Na+, Mg2+, and Ca2+ ions sensitize and activate the capsaicin receptor, TRPV1. At room temperature, increasing extracellular Mg2+ (from 1 to 5 mM) or Na+ (+50 mM) increased ligand-activated currents up to fourfold, and 10 mM Mg2+ reduced the EC50 for activation by capsaicin from 890 to 450 nM. Moreover, concentrations of divalent cations >10 mM directly gate the receptor. These effects occur via electrostatic interactions with two glutamates (E600 and E648) formerly identified as proton-binding residues. Furthermore, phospholipase C-mediated signaling enhances the effects of cations, and physiological concentrations of cations contribute to the bradykinin-evoked activation of TRPV1 and the sensitization of the receptor to heat. Thus, the modulation of TRPV1 by cationic strength may contribute to inflammatory pain signaling.
Figures

References
-
- Agarwal A, Dhiraj S, Raza M, Pandey R, Pandey CK, Singh PK, Singh U, Gupta D (2004) Vein pretreatment with magnesium sulfate to prevent pain on injection of propofol is not justified. Can J Anaesth 51: 130-133. - PubMed
-
- Ahern GP (2003) Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem 278: 30429-30434. - PubMed
-
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD (2003) Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 39: 497-511. - PubMed
-
- Anderson SD (1984) Is there a unifying hypothesis for exercise-induced asthma? J Allergy Clin Immunol 73: 660-665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
