Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells
- PMID: 15917474
- PMCID: PMC2707939
- DOI: 10.1634/stemcells.2004-0365
Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells
Abstract
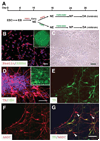
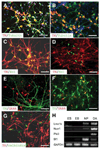
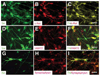
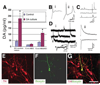
How dopamine (DA) neuronal subtypes are specified remains unknown. In this study we show a robust generation of functional DA neurons from human embryonic stem cells (hESCs) through a specific sequence of application of fibroblast growth factor 8 (FGF8) and sonic hedgehog (SHH). Treatment of hESC-derived Sox1+ neuroepithelial cells with FGF8 and SHH resulted in production of tyrosine hydroxylase (TH)-positive neurons that were mostly bipolar cells, coexpression with gamma-aminobutyric acid, and lack of midbrain marker engrailed 1 (En1) expression. However, FGF8 treatment of precursor cells before Sox1 expression led to the generation of a similar proportion of TH+ neurons characteristic of midbrain projection DA neurons with large cell bodies and complex processes and coexpression of En1. This suggests that one mechanism of generating neuronal subtypes is temporal availability of morphogens to a specific group of precursors. The in vitro-generated DA neurons were electrophysiologically active and released DA in an activity-dependent manner. They may thus provide a renewable source of functional human DA neurons for drug screening and development of sustainable therapeutics for disorders affecting the DA system.
Figures

References
-
- Bjorklund A, Lindvall O. Dopamine-containing systems in the CNS. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Vol. 2. Amsterdam: Elsevier Science publishers; 1984. pp. 55–111. Classical Transmitters in the CNS.
-
- Gall CM, Hendry SH, Seroogy KB, et al. Evidence for coexistence of GABA and dopamine in neurons of the rat olfactory bulb. J Comp Neurol. 1987;266:307–318. - PubMed
-
- Kosaka T, Kosaka K, Hataguchi Y, et al. Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunore-activities in various brain regions of the rat. Exp Brain Res. 1987;66:191–210. - PubMed
-
- Zetterstrom RH, Solomin L, Jansson L, et al. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

