IMP dehydrogenase from the protozoan parasite Toxoplasma gondii
- PMID: 15917510
- PMCID: PMC1140536
- DOI: 10.1128/AAC.49.6.2172-2179.2005
IMP dehydrogenase from the protozoan parasite Toxoplasma gondii
Abstract
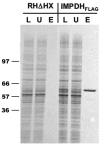
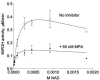
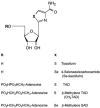
The opportunistic apicomplexan parasite Toxoplasma gondii damages fetuses in utero and threatens immunocompromised individuals. The toxicity associated with standard antitoxoplasmal therapies, which target the folate pathway, underscores the importance of examining alternative pharmacological strategies. Parasitic protozoa cannot synthesize purines de novo; consequently, targeting purine salvage enzymes is a plausible pharmacological strategy. Several enzymes critical to purine metabolism have been studied in T. gondii, but IMP dehydrogenase (IMPDH), which catalyzes the conversion of IMP to XMP, has yet to be characterized. Thus, we have cloned the gene encoding this enzyme in T. gondii. Northern blot analysis shows that two IMPDH transcripts are present in T. gondii tachyzoites. The larger transcript contains an open reading frame of 1,656 nucleotides whose deduced protein sequence consists of 551 amino acids (TgIMPDH). The shorter transcript is an alternative splice product that generates a 371-amino-acid protein lacking the active-site flap (TgIMPDH-S). When TgIMPDH is expressed as a recombinant protein fused to a FLAG tag, the fusion protein localizes to the parasite cytoplasm. Immunoprecipitation with anti-FLAG was employed to purify recombinant TgIMPDH, which converts IMP to XMP as expected. Mycophenolic acid is an uncompetitive inhibitor relative to NAD+, with a intercept inhibition constant (Kii) of 0.03+/-0.004 microM. Tiazofurin and its seleno analog were not inhibitory to the purified enzyme, but adenine dinucleotide analogs such as TAD and the nonhydrolyzable beta-methylene derivatives of TAD or SAD were inhibitory, with Kii values 13- to 60-fold higher than that of mycophenolic acid.
Figures



Similar articles
-
Mycophenolic acid and thiazole adenine dinucleotide inhibition of Tritrichomonas foetus inosine 5'-monophosphate dehydrogenase: implications on enzyme mechanism.Biochemistry. 1990 Jan 30;29(4):849-54. doi: 10.1021/bi00456a001. Biochemistry. 1990. PMID: 1971185
-
Cloning, sequencing, and structural analysis of the DNA encoding inosine monophosphate dehydrogenase (EC 1.1.1.205) from Tritrichomonas foetus.Exp Parasitol. 1994 Feb;78(1):101-12. doi: 10.1006/expr.1994.1010. Exp Parasitol. 1994. PMID: 7905423
-
The roles of conserved carboxylate residues in IMP dehydrogenase and identification of a transition state analog.Biochemistry. 1997 Oct 28;36(43):13365-73. doi: 10.1021/bi9714161. Biochemistry. 1997. PMID: 9341229
-
Nucleoside and non-nucleoside IMP dehydrogenase inhibitors as antitumor and antiviral agents.Curr Med Chem. 1999 Jul;6(7):599-614. Curr Med Chem. 1999. PMID: 10390603 Review.
-
The structure of inosine 5'-monophosphate dehydrogenase and the design of novel inhibitors.Immunopharmacology. 2000 May;47(2-3):163-84. doi: 10.1016/s0162-3109(00)00193-4. Immunopharmacology. 2000. PMID: 10878288 Review.
Cited by
-
Mycophenolic Acid and Its Derivatives as Potential Chemotherapeutic Agents Targeting Inosine Monophosphate Dehydrogenase in Trypanosoma congolense.Antimicrob Agents Chemother. 2016 Jun 20;60(7):4391-3. doi: 10.1128/AAC.02816-15. Print 2016 Jul. Antimicrob Agents Chemother. 2016. PMID: 27139487 Free PMC article.
-
Targeting purine and pyrimidine metabolism in human apicomplexan parasites.Curr Drug Targets. 2007 Jan;8(1):31-47. doi: 10.2174/138945007779315524. Curr Drug Targets. 2007. PMID: 17266529 Free PMC article. Review.
-
Effect of urea and squaramide IMPDH inhibitors on C. parvum: in vitro trial design impacts the assessment of drug efficacy.Int J Parasitol Drugs Drug Resist. 2025 Aug;28:100592. doi: 10.1016/j.ijpddr.2025.100592. Epub 2025 Apr 15. Int J Parasitol Drugs Drug Resist. 2025. PMID: 40319744 Free PMC article.
-
New Nucleic Base-Tethered Trithiolato-Bridged Dinuclear Ruthenium(II)-Arene Compounds: Synthesis and Antiparasitic Activity.Molecules. 2022 Nov 24;27(23):8173. doi: 10.3390/molecules27238173. Molecules. 2022. PMID: 36500266 Free PMC article.
-
MYST family lysine acetyltransferase facilitates ataxia telangiectasia mutated (ATM) kinase-mediated DNA damage response in Toxoplasma gondii.J Biol Chem. 2010 Apr 9;285(15):11154-61. doi: 10.1074/jbc.M109.066134. Epub 2010 Feb 16. J Biol Chem. 2010. PMID: 20159970 Free PMC article.
References
-
- Boritzki, T. J., D. A. Berry, J. A. Besserer, P. D. Cook, D. W. Fry, W. R. Leopold, and R. C. Jackson. 1985. Biochemical and antitumor activity of tiazofurin and its selenium analog (2-beta-d-ribofuranosyl-4-selenazolecarboxamide). Biochem. Pharmacol. 34:1109-1114. - PubMed
-
- Chaudhary, K., J. A. Darling, L. M. Fohl, W. J. Sullivan, Jr., R. G. Donald, E. R. Pfefferkorn, B. Ullman, and D. S. Roos. 2004. Purine salvage pathways in the apicomplexan parasite Toxoplasma gondii. J. Biol. Chem. 279:31221-31227. - PubMed
-
- Chiang, C. W., N. Carter, W. J. Sullivan, Jr., R. G. Donald, D. S. Roos, F. N. Naguib, M. H. el Kouni, B. Ullman, and C. M. Wilson. 1999. The adenosine transporter of Toxoplasma gondii. Identification by insertional mutagenesis, cloning, and recombinant expression. J. Biol. Chem. 274:35255-35261. - PubMed
-
- Digits, J. A., and L. Hedstrom. 2000. Drug selectivity is determined by coupling across the NAD+ site of IMP dehydrogenase. Biochemistry 39:1771-1777. - PubMed
-
- Digits, J. A., and L. Hedstrom. 1999. Kinetic mechanism of Tritrichomonas foetus inosine 5′-monophosphate dehydrogenase. Biochemistry 38:2295-2306. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

