TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates
- PMID: 15917527
- PMCID: PMC1140553
- DOI: 10.1128/AAC.49.6.2314-2321.2005
TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates
Abstract
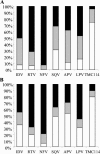
The purpose of this study was to characterize the antiviral activity, cytotoxicity, and mechanism of action of TMC114, a novel human immunodeficiency virus type 1 (HIV-1) protease inhibitor (PI). TMC114 exhibited potent anti-HIV activity with a 50% effective concentration (EC50) of 1 to 5 nM and a 90% effective concentration of 2.7 to 13 nM. TMC114 exhibited no cytotoxicity at concentrations up to 100 muM (selectivity index, >20,000). All viruses in a panel of 19 recombinant clinical isolates carrying multiple protease mutations and demonstrating resistance to an average of five other PIs, were susceptible to TMC114, defined as a fold change in EC50 of <4. TMC114 was also effective against the majority of 1,501 PI-resistant recombinant viruses derived from recent clinical samples, with EC50s of <10 nM for 75% of the samples. In sequential passage experiments using HIV-1 LAI, two mutations (R41T and K70E) were selected. One selected virus showed a 10-fold reduction in susceptibility to TMC114, but <10-fold reductions in susceptibility to the current PIs (atazanavir was not assessed), except saquinavir. However, when the selected mutations were introduced into a laboratory strain by site-directed mutagenesis, they had no effect on susceptibility to TMC114 or other PIs. There was no evidence of antagonism between TMC114 and any currently available PIs or reverse transcriptase inhibitors. Combinations with ritonavir, nelfinavir, and amprenavir showed some evidence of synergy. These results suggest that TMC114 is a potential candidate for the treatment of both naive and PI-experienced patients with HIV.
Figures


References
-
- Balotta, C., G. Facchi, M. Violin, S. Van Dooren, A. Cozzi-Lepri, F. Forbici, A. Bertoli, C. Riva, D. Senese, P. Caramello, G. Carnevale, G. Rizzardini, L. Cremonini, L. Monno, G. Rezza, C. F. Perno, G. Ippolito, A. d'Arminio-Monforte, A. M. Vandamme, and M. Moroni. 2001. Increasing prevalence of non-clade B HIV-1 strains in heterosexual men and women, as monitored by analysis of reverse transcriptase and protease sequences. J Acquir. Immune Defic. Syndr. 27:499-505. - PubMed
-
- Bartlett, J. A., R. DeMasi, J. Quinn, C. Moxham, and F. Rousseau. 2001. Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS 15:1369-1377. - PubMed
-
- Bilello, J. A., P. A. Bilello, K. Stellrecht, J. Leonard, D. W. Norbeck, D. J. Kempf, T. Robins, and G. L. Drusano. 1996. Human serum alpha 1 acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of A-80987, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob. Agents Chemother. 40:1491-1497. - PMC - PubMed
-
- Bilello, J. A., and G. L. Drusano. 1996. Relevance of plasma protein binding to antiviral activity and clinical efficacy of inhibitors of human immunodeficiency virus protease. J. Infect. Dis. 173:1524-1526. - PubMed
-
- Craig, J. C., I. B. Duncan, D. Hockley, C. Grief, N. A. Roberts, and J. S. Mills. 1991. Antiviral properties of Ro 31-8959, an inhibitor of human immunodeficiency virus (HIV) proteinase. Antiviral Res. 16:295-305. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

