Antifungal protein PAF severely affects the integrity of the plasma membrane of Aspergillus nidulans and induces an apoptosis-like phenotype
- PMID: 15917545
- PMCID: PMC1140496
- DOI: 10.1128/AAC.49.6.2445-2453.2005
Antifungal protein PAF severely affects the integrity of the plasma membrane of Aspergillus nidulans and induces an apoptosis-like phenotype
Abstract
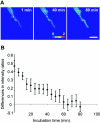
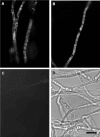
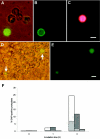
The small, basic, and cysteine-rich antifungal protein PAF is abundantly secreted into the supernatant by the beta-lactam producer Penicillium chrysogenum. PAF inhibits the growth of various important plant and zoopathogenic filamentous fungi. Previous studies revealed the active internalization of the antifungal protein and the induction of multifactorial detrimental effects, which finally resulted in morphological changes and growth inhibition in target fungi. In the present study, we offer detailed insights into the mechanism of action of PAF and give evidence for the induction of a programmed cell death-like phenotype. We proved the hyperpolarization of the plasma membrane in PAF-treated Aspergillus nidulans hyphae by using the aminonaphtylethenylpyridinium dye di-8-ANEPPS. The exposure of phosphatidylserine on the surface of A. nidulans protoplasts by Annexin V staining and the detection of DNA strand breaks by TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) gave evidence for a PAF-induced apoptotic-like mechanism in A. nidulans. The localization of reactive oxygen species (ROS) by dichlorodihydrofluorescein diacetate and the abnormal cellular ultrastructure analyzed by transmission electron microscopy suggested that ROS-elicited membrane damage and the disintegration of mitochondria played a major role in the cytotoxicity of PAF. Finally, the reduced PAF sensitivity of A. nidulans strain FGSC1053, which carries a dominant-interfering mutation in fadA, supported our assumption that G-protein signaling was involved in PAF-mediated toxicity.
Figures



References
-
- Arndt-Jovin, D. J., and T. M. Jovin. 1989. Fluorescence labeling and microscopy of DNA. Methods Cell Biol. 30:417-448. - PubMed
-
- Beach, J. M., E. D. McGahren, J. Xia, and B. R. Duling. 1996. Ratiometric measurement of endothelial depolarization in arterioles with a potential-sensitive dye. Am. J. Physiol. 270:2216-2227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

