Role of phospholipase D and diacylglycerol in activating constitutive TRPC-like cation channels in rabbit ear artery myocytes
- PMID: 15919706
- PMCID: PMC1464787
- DOI: 10.1113/jphysiol.2005.090852
Role of phospholipase D and diacylglycerol in activating constitutive TRPC-like cation channels in rabbit ear artery myocytes
Abstract
Previously we have described a constitutively active Ca2+-permeable non-selective cation channel in freshly dispersed rabbit ear artery myocytes that has similar properties to canonical transient receptor potential (TRPC) channel proteins. In the present study we have investigated the transduction pathways responsible for stimulating constitutive channel activity in these myocytes. Application of the pharmacological inhibitors of phosphatidylcholine-phospholipase D (PC-PLD), butan-1-ol and C2 ceramide, produced marked inhibition of constitutive channel activity in cell-attached patches and also butan-1-ol produced pronounced suppression of resting membrane conductance measured with whole-cell recording whereas the inactive isomer butan-2-ol had no effect on constitutive whole-cell or channel activity. In addition butan-1-ol had no effect on channel activity evoked by the diacylglycerol (DAG) analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG). Inhibitors of PC-phospholipase C (PC-PLC) and phospholipase A2 (PLA2) had no effect on constitutive channel activity. Application of a purified PC-PLD enzyme and its metabolite phosphatidic acid to inside-out patches markedly increased channel activity. The phosphatidic acid phosphohydrolase (PAP) inhibitor dl-propranolol also inhibited constitutive and phosphatidic acid-induced increases in channel activity but had no effect on OAG-evoked responses. The DAG lipase and DAG kinase inhibitors, RHC80267 and R59949 respectively, which inhibit DAG metabolism, produced transient increases in channel activity which were mimicked by relatively high concentrations (40 microm) of OAG. The protein kinase C (PKC) inhibitor chelerythrine did not prevent channel activation by OAG but blocked the secondary inhibitory response of OAG. It is proposed that endogenous DAG is involved in the activation of channel activity and that its effects on channel activity are concentration-dependent with higher concentrations of DAG also inhibiting channel activity through activation of PKC. This study indicates that constitutive cation channel activity in ear artery myocytes is mediated by DAG which is generated by PC-PLD via phosphatidic acid which represents a novel activation pathway of cation channels in vascular myocytes.
Figures

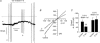


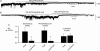

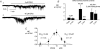
References
-
- Abousalham A, Liossis C, O'Brien L, Brindley DN. Cell Permeable ceramides prevent the activation of phospholipase D by ADP-ribosylation factor and RhoA. J Biol Chem. 1997;272:1069–1075. - PubMed
-
- Aburto T, Jinsi A, Zhu Q, Deth RC. Involvement of protein kinase C activation in alpha2-adrenoceptor-mediated contractions of rabbit saphenous vein. Eur J Pharmacol. 1995;302:35–44. - PubMed
-
- Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995;270:445–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

