Inverted photocurrent responses from amphibian rod photoreceptors: role of membrane voltage in response recovery
- PMID: 15919708
- PMCID: PMC1464743
- DOI: 10.1113/jphysiol.2005.090258
Inverted photocurrent responses from amphibian rod photoreceptors: role of membrane voltage in response recovery
Abstract
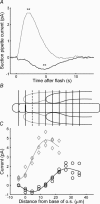
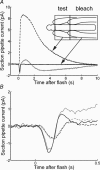
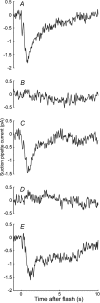
We recorded photocurrent responses of retinal rods isolated from cane toads Bufo marinus and clawed frogs Xenopus laevis. With the outer segment drawn part way into the suction pipette, presentation of flashes to the base of the outer segment (outside the pipette) elicited a slow inverted response. Stimulation of the same region, with the outer segment drawn fully in, gave a response of conventional polarity. For moderate to bright flashes a fast transient preceded the slow inverted response. Upon bleaching the tip of the outer segment, the slow inverted response was abolished but the fast initial transient remained, and we attribute this fast component to a capacitive current. Experiments employing simultaneous whole-cell patch-clamp and suction pipette recording revealed that both the fast and slow components of the inverted responses were absent in voltage-clamped cells. In current-clamped cells the slow inverted current response was delayed substantially with respect to the voltage response. We present a computational model for the slow component, in which hyperpolarization leads to increased activity of the Na+ -Ca2+, K+ exchanger, hence lowering the cytoplasmic Ca2+ concentration, activating guanylyl cyclase, raising cyclic GMP concentration, opening cyclic nucleotide-gated channels, and increasing circulating current in the unstimulated region. For the measured voltage response to stimulation of the base, we solve these equations to predict the photocurrent in the tip, and obtain an adequate explanation of the inverted responses. Our work suggests a novel role for membrane voltage in accelerating the inactivation phase of the response to light.
Figures




Similar articles
-
Differences in calcium homeostasis between retinal rod and cone photoreceptors revealed by the effects of voltage on the cGMP-gated conductance in intact cells.J Gen Physiol. 1994 Nov;104(5):909-40. doi: 10.1085/jgp.104.5.909. J Gen Physiol. 1994. PMID: 7876828 Free PMC article.
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
-
Gating by cyclic GMP and voltage in the alpha subunit of the cyclic GMP-gated channel from rod photoreceptors.J Gen Physiol. 1999 Oct;114(4):477-90. doi: 10.1085/jgp.114.4.477. J Gen Physiol. 1999. PMID: 10498668 Free PMC article.
-
Characterization of guanylate cyclase activity in single retinal rod outer segments.J Gen Physiol. 1995 Nov;106(5):863-90. doi: 10.1085/jgp.106.5.863. J Gen Physiol. 1995. PMID: 8648296 Free PMC article.
-
Binding of the retinal rod Na+/Ca2+-K+ exchanger to the cGMP-gated channel indicates local Ca(2+)-signaling in vertebrate photoreceptors.Ann N Y Acad Sci. 2002 Nov;976:325-34. doi: 10.1111/j.1749-6632.2002.tb04755.x. Ann N Y Acad Sci. 2002. PMID: 12502575 Review.
Cited by
-
Ectopic expression of cone-specific G-protein-coupled receptor kinase GRK7 in zebrafish rods leads to lower photosensitivity and altered responses.J Physiol. 2011 May 1;589(Pt 9):2321-48. doi: 10.1113/jphysiol.2010.204156. Epub 2011 Mar 8. J Physiol. 2011. PMID: 21486791 Free PMC article.
-
Loss of the K+ channel Kv2.1 greatly reduces outward dark current and causes ionic dysregulation and degeneration in rod photoreceptors.J Gen Physiol. 2021 Feb 1;153(2):e202012687. doi: 10.1085/jgp.202012687. J Gen Physiol. 2021. PMID: 33502442 Free PMC article.
-
Complementary conductance changes by IKx and Ih contribute to membrane impedance stability during the rod light response.Channels (Austin). 2009 Sep-Oct;3(5):301-7. doi: 10.4161/chan.3.5.9454. Epub 2009 Sep 7. Channels (Austin). 2009. PMID: 19713736 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

