Cell type-specific action of seizure-induced intracellular zinc accumulation in the rat hippocampus
- PMID: 15919712
- PMCID: PMC1464793
- DOI: 10.1113/jphysiol.2005.089458
Cell type-specific action of seizure-induced intracellular zinc accumulation in the rat hippocampus
Abstract
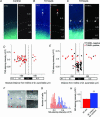
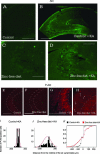
Increased levels of intracellular zinc have been implicated in neuronal cell death in ischaemia, epilepsy and traumatic brain damage. However, decreases in zinc levels also lead to increased neuronal death and lowered seizure threshold. In the present study we investigated the physiological role of zinc in neurodegeneration and protection following epileptic seizures. Cells located in the strata oriens and lucidum of the CA3 region accumulated high concentrations of zinc and died. A decrease in zinc level could prevent the death of these neurones after seizures. Most of these cells were GABAergic interneurones. In contrast, neurones in the CA3 pyramidal cell layer accumulated moderate amounts of zinc and survived. Zinc chelation led to an increase in the mortality rate of these cells. Furthermore, in these cells low concentrations of intracellular zinc activated Akt (protein kinase B), thus providing protection against neurodegeneration. These results demonstrate that intracellularly accumulated zinc can be neurotoxic or neuroprotective depending on its concentration. This dual action is cell type specific.
Figures





Similar articles
-
Extracellular chelation of zinc does not affect hippocampal excitability and seizure-induced cell death in rats.J Physiol. 2007 Jan 1;578(Pt 1):275-89. doi: 10.1113/jphysiol.2006.121848. Epub 2006 Nov 9. J Physiol. 2007. PMID: 17095563 Free PMC article.
-
Erythropoietin prevents zinc accumulation and neuronal death after traumatic brain injury in rat hippocampus: in vitro and in vivo studies.Brain Res. 2009 Sep 15;1289:96-105. doi: 10.1016/j.brainres.2009.07.015. Epub 2009 Jul 15. Brain Res. 2009. PMID: 19615349
-
Neurotoxic zinc translocation into hippocampal neurons is inhibited by hypothermia and is aggravated by hyperthermia after traumatic brain injury in rats.J Cereb Blood Flow Metab. 2006 Feb;26(2):161-9. doi: 10.1038/sj.jcbfm.9600176. J Cereb Blood Flow Metab. 2006. PMID: 15988476
-
Adrenalectomy causes loss of zinc ions in zinc-enriched (ZEN) terminals and decreases seizure-induced neuronal death.Brain Res. 2001 Mar 23;895(1-2):25-32. doi: 10.1016/s0006-8993(01)01996-5. Brain Res. 2001. PMID: 11259756
-
c-Fos, N-methyl-d-aspartate receptor 2C, GABA-A-alpha1 immonoreactivity, seizure latency and neuronal injury following single or recurrent neonatal seizures in hippocampus of Wistar rat.Neurosci Lett. 2005 May 20-27;380(1-2):149-54. doi: 10.1016/j.neulet.2005.01.043. Epub 2005 Feb 3. Neurosci Lett. 2005. PMID: 15854768
Cited by
-
The neuroprotective effects of icariin on ageing, various neurological, neuropsychiatric disorders, and brain injury induced by radiation exposure.Aging (Albany NY). 2022 Feb 14;14(3):1562-1588. doi: 10.18632/aging.203893. Epub 2022 Feb 14. Aging (Albany NY). 2022. PMID: 35165207 Free PMC article.
-
Seizure enhances SUMOylation and zinc-finger transcriptional repression in neuronal nuclei.iScience. 2023 Aug 23;26(9):107707. doi: 10.1016/j.isci.2023.107707. eCollection 2023 Sep 15. iScience. 2023. PMID: 37694138 Free PMC article.
-
Insight into glutamate excitotoxicity from synaptic zinc homeostasis.Int J Alzheimers Dis. 2010 Dec 20;2011:491597. doi: 10.4061/2011/491597. Int J Alzheimers Dis. 2010. PMID: 21234391 Free PMC article.
-
Chemical blocking of zinc ions in CNS increases neuronal damage following traumatic brain injury (TBI) in mice.PLoS One. 2010 Apr 9;5(4):e10131. doi: 10.1371/journal.pone.0010131. PLoS One. 2010. PMID: 20396380 Free PMC article.
-
Role of Maternal Microbiota and Nutrition in Early-Life Neurodevelopmental Disorders.Nutrients. 2021 Oct 9;13(10):3533. doi: 10.3390/nu13103533. Nutrients. 2021. PMID: 34684534 Free PMC article. Review.
References
-
- Aizenman E, Stout AK, Hartnett KA, Dineley KE, Mclaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–1888. - PubMed
-
- Allington C, Shamovsky IL, Ross GM, Riopelle RJ. Zinc inhibits p75NTR-mediated apoptosis in chick neural retina. Cell Death Differ. 2001;8:451–456. - PubMed
-
- Andrews JC, Nolan JP, Hammerstedt RH, Bavister BD. Characterization of N-(6-methoxy-8-quinolyl)-p-toluenesulfonamide for the detection of zinc in living sperm cells. Cytometry. 1995;21:153–159. - PubMed
-
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. - PubMed
-
- Berendji D, Kolb-Bachofen V, Meyer KL, Grapenthin O, Weber H, Wahn V, Kroncke KD. Nitric oxide mediates intracytoplasmic and intranuclear zinc release. FEBS Lett. 1997;405:37–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

