The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-gamma2 isoform
- PMID: 15919792
- PMCID: PMC4304004
- DOI: 10.2337/diabetes.54.6.1706
The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-gamma2 isoform
Abstract
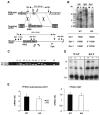
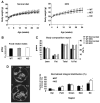
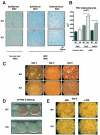
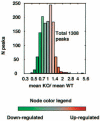
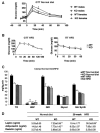
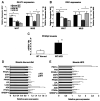
The nuclear receptor peroxisome proliferator-activated receptor-gamma (PPARgamma) is critically required for adipogenesis. PPARgamma exists as two isoforms, gamma1 and gamma2. PPARgamma2 is the more potent adipogenic isoform in vitro and is normally restricted to adipose tissues, where it is regulated more by nutritional state than PPARgamma1. To elucidate the relevance of the PPARgamma2 in vivo, we generated a mouse model in which the PPARgamma2 isoform was specifically disrupted. Despite similar weight, body composition, food intake, energy expenditure, and adipose tissue morphology, male mice lacking the gamma2 isoform were more insulin resistant than wild-type animals when fed a regular diet. These results indicate that insulin resistance associated with ablation of PPARgamma2 is not the result of lipodystrophy and suggests a specific role for PPARgamma2 in maintaining insulin sensitivity independently of its effects on adipogenesis. Furthermore, PPARgamma2 knockout mice fed a high-fat diet did not become more insulin resistant than those on a normal diet, despite a marked increase in their mean adipocyte cell size. These findings suggest that PPARgamma2 is required for the maintenance of normal insulin sensitivity in mice but also raises the intriguing notion that PPARgamma2 may be necessary for the adverse effects of a high-fat diet on carbohydrate metabolism.
Figures

References
-
- Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology. 2001;142:4195–4202. - PubMed
-
- Werman A, Hollenberg A, Solanes G, Bjorbaek C, Vidal-Puig AJ, Flier JS. Ligand-independent activation domain in the N terminus of peroxisome proliferator-activated receptor γ (PPARγ): differential activity of PPARγ1 and -2 isoforms and influence of insulin. J Biol Chem. 1997;272:20230–20235. - PubMed
-
- Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, Tuomi T, Gaudet D, Hudson TJ, Daly M, Groop L, Lander ES. The common PPARγ Pro12Alapolymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26:76–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

