Induction of Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen by the lytic transactivator RTA: a novel mechanism for establishment of latency
- PMID: 15919901
- PMCID: PMC1143691
- DOI: 10.1128/JVI.79.12.7453-7465.2005
Induction of Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen by the lytic transactivator RTA: a novel mechanism for establishment of latency
Abstract
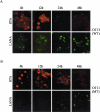
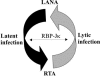
Kaposi's sarcoma-associated herpesvirus (KSHV) is the etiological agent contributing to development of Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman desease. Following primary infection, latency is typically established. However, the mechanism by which KSHV establishes latency is not understood. We have reported that the latency-associated nuclear antigen (LANA) can repress RTA (for replication and transcription activator) expression by down-regulating its promoter. In this study, we show that RTA is associated with the virion particle. We also show that RTA can activate the LANA promoter and induce LANA expression in transient reporter assays. Additionally, the transcription of RTA correlates with LANA expression in the early stages of de novo infection of KSHV, and induction of LANA transcription is responsive to induction of RTA with an inducible system. This induction in LANA transcription was dependent on recombination signal sequence binding protein Jkappa (RBP-Jkappa), as a RBP-Jkappa-deficient cell line was significantly delayed and inefficient in LANA transcription with expression of RTA. These studies suggest that RTA contributes to establishment of KSHV latency by activating LANA expression in the early stages of infection by utilizing the major effector of the Notch signaling pathway RBP-Jkappa. This describes a feedback mechanism by which LANA and RTA can regulate each other and is likely to be a key event in the establishment of KSHV latency.
Figures

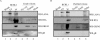

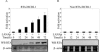



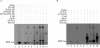
Similar articles
-
Kaposi's sarcoma-associated herpesvirus reactivation is regulated by interaction of latency-associated nuclear antigen with recombination signal sequence-binding protein Jkappa, the major downstream effector of the Notch signaling pathway.J Virol. 2005 Mar;79(6):3468-78. doi: 10.1128/JVI.79.6.3468-3478.2005. J Virol. 2005. PMID: 15731241 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency.J Virol. 2004 Jun;78(12):6585-94. doi: 10.1128/JVI.78.12.6585-6594.2004. J Virol. 2004. PMID: 15163750 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen modulates K1 expression through its cis-acting elements within the terminal repeats.J Virol. 2006 Apr;80(7):3445-58. doi: 10.1128/JVI.80.7.3445-3458.2006. J Virol. 2006. PMID: 16537612 Free PMC article.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
-
KSHV Genome Replication and Maintenance in Latency.Adv Exp Med Biol. 2018;1045:299-320. doi: 10.1007/978-981-10-7230-7_14. Adv Exp Med Biol. 2018. PMID: 29896673 Review.
Cited by
-
The RBP-Jκ binding sites within the RTA promoter regulate KSHV latent infection and cell proliferation.PLoS Pathog. 2012 Jan;8(1):e1002479. doi: 10.1371/journal.ppat.1002479. Epub 2012 Jan 12. PLoS Pathog. 2012. PMID: 22253595 Free PMC article.
-
KSHV encoded LANA recruits Nucleosome Assembly Protein NAP1L1 for regulating viral DNA replication and transcription.Sci Rep. 2016 Sep 7;6:32633. doi: 10.1038/srep32633. Sci Rep. 2016. PMID: 27599637 Free PMC article.
-
Exploring the DNA binding interactions of the Kaposi's sarcoma-associated herpesvirus lytic switch protein by selective amplification of bound sequences in vitro.J Virol. 2006 Mar;80(6):2958-67. doi: 10.1128/JVI.80.6.2958-2967.2006. J Virol. 2006. PMID: 16501105 Free PMC article.
-
Guanylate-Binding Protein 1 Inhibits Nuclear Delivery of Kaposi's Sarcoma-Associated Herpesvirus Virions by Disrupting Formation of Actin Filament.J Virol. 2017 Jul 27;91(16):e00632-17. doi: 10.1128/JVI.00632-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28592529 Free PMC article.
-
Molecular biology of Kaposi's sarcoma-associated herpesvirus and related oncogenesis.Adv Virus Res. 2010;78:87-142. doi: 10.1016/B978-0-12-385032-4.00003-3. Adv Virus Res. 2010. PMID: 21040832 Free PMC article. Review.
References
-
- Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, E. A. Mesri, and M. C. Gerhengorn. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. - PubMed
-
- Bais, C., A. Van Geelen, P. Eroles, A. Mutlu, C. Chiozzini, S. Dias, R. L. Silverstein, S. Rafii, and E. A. Mesri. 2003. Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/ KDR. Cancer Cell 3:131-143. - PubMed
-
- Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

