Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity
- PMID: 15919903
- PMCID: PMC1143681
- DOI: 10.1128/JVI.79.12.7478-7491.2005
Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity
Abstract
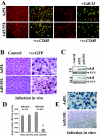
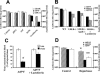
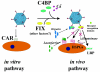
Adenoviruses (Ad) are efficient vehicles for gene delivery in vitro and in vivo. Therefore, they are a promising tool in gene therapy, particularly in the treatment of cancer and cardiovascular diseases. However, preclinical and clinical studies undertaken during the last decade have revealed a series of problems that limit both the safety and efficacy of Ad vectors, specifically after intravenous application. Major obstacles to clinical use include innate toxicity and Ad sequestration by nontarget tissues. The factors and mechanisms underlying these processes are poorly understood. The majority of intravenously injected Ad particles are sequestered by the liver, which in turn causes an inflammatory response characterized by acute transaminitis and vascular damage. Here, we describe a novel pathway that is used by Ad for infection of hepatocytes and Kupffer cells upon intravenous virus application in mice. We found that blood factors play a major role in targeting Ad vectors to hepatic cells. We demonstrated that coagulation factor IX and complement component C4-binding protein can bind the Ad fiber knob domain and provide a bridge for virus uptake through cell surface heparan sulfate proteoglycans and low-density lipoprotein receptor-related protein. An Ad vector, Ad5mut, which contained mutations in the fiber knob domain ablating blood factor binding, demonstrated significantly reduced infection of liver cells and liver toxicity in vivo. This study contributes to a better understanding of adenovirus-host interactions for intravenously applied vectors. It also provides a rationale for novel strategies to target adenovirus vector to specific tissues and to reduce virus-associated toxicity after systemic application.
Figures




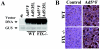



References
-
- Alemany, R., and D. T. Curiel. 2001. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 8:1347-1353. - PubMed
-
- Ben-Gary, H., R. L. McKinney, T. Rosengart, M. L. Lesser, and R. G. Crystal. 2002. Systemic interleukin-6 responses following administration of adenovirus gene transfer vectors to humans by different routes. Mol. Ther. 6:287-297. - PubMed
-
- Bernt, K. M., D. S. Steinwaerder, S. Ni, Z. Y. Li, S. R. Roffler, and A. Lieber. 2002. Enzyme-activated prodrug therapy enhances tumor-specific replication of adenovirus vectors. Cancer Res. 62:6089-6098. - PubMed
-
- Branchereau, S., D. Calise, and N. Ferry. 1994. Factors influencing retroviral-mediated gene transfer into hepatocytes in vivo. Hum. Gene Ther. 5:803-808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

