Abortive versus productive viral infection of dendritic cells with a paramyxovirus results in differential upregulation of select costimulatory molecules
- PMID: 15919909
- PMCID: PMC1143689
- DOI: 10.1128/JVI.79.12.7544-7557.2005
Abortive versus productive viral infection of dendritic cells with a paramyxovirus results in differential upregulation of select costimulatory molecules
Abstract
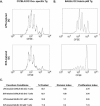
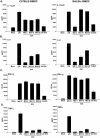
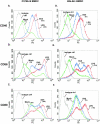
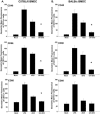
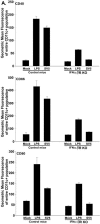
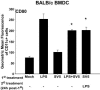
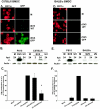
Dendritic cells are the most potent antigen-presenting cell for priming naive T cells. Optimal activation of T cells requires that dendritic cells undergo a process of maturation resulting in the increased expression of costimulatory molecules, such as CD40, CD86, and CD80, and the production of cytokines. In this study we analyzed the effect of infection of dendritic cells obtained from two strains of mice, BALB/c and C57BL/6, with the paramyxovirus simian virus 5 (SV5). Our results show that C57BL/6 bone marrow-derived dendritic cells (BMDC) are much more permissive to infection with SV5 at a multiplicity of infection (MOI) of 10 PFU/cell compared to BALB/c BMDC, as determined by the production of viral proteins and progeny. However, infection of BALB/c BMDC with a higher MOI of 50 PFU/cell resulted in a productive infection with the production of significant amounts of viral proteins and progeny. Regardless of the permissivity to infection, both BALB/c and C57BL/6 BMDC efficiently upregulated CD40 and CD86. However, CD80 upregulation correlated with the level of expression of viral proteins and the production of viral progeny. While secreted alpha/beta interferon was required for increased expression of all three molecules, optimal CD80 expression was dependent on an additional signal provided by a productive viral infection. These findings provide evidence that the signals controlling the expression of costimulatory molecules following viral infection are distinct. Further, they suggest that the amount of virus encountered and/or the permissivity of a dendritic cell to infection can alter the resulting maturation phenotype and functional capacity of the infected dendritic cell.
Figures
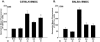
Similar articles
-
Distinct pathways for signaling maturation in macrophages and dendritic cells after infection with paramyxovirus simian virus 5.Viral Immunol. 2007 Spring;20(1):76-87. doi: 10.1089/vim.2006.0070. Viral Immunol. 2007. PMID: 17425423
-
A simian virus 5 (SV5) P/V mutant is less cytopathic than wild-type SV5 in human dendritic cells and is a more effective activator of dendritic cell maturation and function.J Virol. 2006 Apr;80(7):3416-27. doi: 10.1128/JVI.80.7.3416-3427.2006. J Virol. 2006. PMID: 16537609 Free PMC article.
-
Vaccinia virus infection of mature dendritic cells results in activation of virus-specific naïve CD8+ T cells: a potential mechanism for direct presentation.Virology. 2007 Mar 15;359(2):349-61. doi: 10.1016/j.virol.2006.09.020. Epub 2006 Oct 20. Virology. 2007. PMID: 17056088 Free PMC article.
-
Limited costimulatory molecule expression on renal tubular epithelial cells impairs T cell activation.Kidney Blood Press Res. 2007;30(6):421-9. doi: 10.1159/000110578. Epub 2007 Oct 26. Kidney Blood Press Res. 2007. PMID: 17975322
-
[Developmental pathway of dendritic cell subsets].Seikagaku. 2007 Dec;79(12):1143-8. Seikagaku. 2007. PMID: 18203455 Review. Japanese. No abstract available.
Cited by
-
Paramyxovirus activation and inhibition of innate immune responses.J Mol Biol. 2013 Dec 13;425(24):4872-92. doi: 10.1016/j.jmb.2013.09.015. Epub 2013 Sep 20. J Mol Biol. 2013. PMID: 24056173 Free PMC article. Review.
-
Functional Impairment of Murine Dendritic Cell Subsets following Infection with Infective Larval Stage 3 of Brugia malayi.Infect Immun. 2016 Dec 29;85(1):e00818-16. doi: 10.1128/IAI.00818-16. Print 2017 Jan. Infect Immun. 2016. PMID: 27799335 Free PMC article.
-
Differences in innate immune responses correlate with differences in murine susceptibility to Chlamydia muridarum pulmonary infection.Immunology. 2010 Apr;129(4):556-66. doi: 10.1111/j.1365-2567.2009.03157.x. Epub 2009 Sep 11. Immunology. 2010. PMID: 20102413 Free PMC article.
-
The in Vitro Inhibitory Effect of Ectromelia Virus Infection on Innate and Adaptive Immune Properties of GM-CSF-Derived Bone Marrow Cells Is Mouse Strain-Independent.Front Microbiol. 2017 Dec 19;8:2539. doi: 10.3389/fmicb.2017.02539. eCollection 2017. Front Microbiol. 2017. PMID: 29312229 Free PMC article.
-
Mouse dendritic cell (DC) influenza virus infectivity is much lower than that for human DCs and is hemagglutinin subtype dependent.J Virol. 2013 Feb;87(3):1916-8. doi: 10.1128/JVI.02980-12. Epub 2012 Nov 28. J Virol. 2013. PMID: 23192878 Free PMC article.
References
-
- Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. 2003. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646-4657. - PMC - PubMed
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. - PubMed
-
- Anderson, D. E., L. J. Ausubel, J. Krieger, P. Hollsberg, G. J. Freeman, and D. A. Hafler. 1997. Weak peptide agonists reveal functional differences in B7-1 and B7-2 costimulation of human T-cell clones. J. Immunol. 159:1669-1675. - PubMed
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

