Proteome analysis of liver cells expressing a full-length hepatitis C virus (HCV) replicon and biopsy specimens of posttransplantation liver from HCV-infected patients
- PMID: 15919910
- PMCID: PMC1143647
- DOI: 10.1128/JVI.79.12.7558-7569.2005
Proteome analysis of liver cells expressing a full-length hepatitis C virus (HCV) replicon and biopsy specimens of posttransplantation liver from HCV-infected patients
Abstract
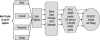
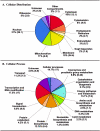
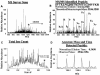
The development of a reproducible model system for the study of hepatitis C virus (HCV) infection has the potential to significantly enhance the study of virus-host interactions and provide future direction for modeling the pathogenesis of HCV. While there are studies describing global gene expression changes associated with HCV infection, changes in the proteome have not been characterized. We report the first large-scale proteome analysis of the highly permissive Huh-7.5 cell line containing a full-length HCV replicon. We detected >4,200 proteins in this cell line, including HCV replicon proteins, using multidimensional liquid chromatographic (LC) separations coupled to mass spectrometry. Consistent with the literature, a comparison of HCV replicon-positive and -negative Huh-7.5 cells identified expression changes of proteins involved in lipid metabolism. We extended these analyses to liver biopsy material from HCV-infected patients where a total of >1,500 proteins were detected from only 2 mug of liver biopsy protein digest using the Huh-7.5 protein database and the accurate mass and time tag strategy. These findings demonstrate the utility of multidimensional proteome analysis of the HCV replicon model system for assisting in the determination of proteins/pathways affected by HCV infection. Our ability to extend these analyses to the highly complex proteome of small liver biopsies with limiting protein yields offers the unique opportunity to begin evaluating the clinical significance of protein expression changes associated with HCV infection.
Figures


Similar articles
-
Proteome analysis of human liver carcinoma Huh7 cells harboring hepatitis C virus subgenomic replicon.Proteomics. 2006 Jan;6(2):519-27. doi: 10.1002/pmic.200500233. Proteomics. 2006. PMID: 16317778
-
Hepatitis C virus core protein stimulates hepatocyte growth: correlation with upregulation of wnt-1 expression.Hepatology. 2005 May;41(5):1096-105. doi: 10.1002/hep.20668. Hepatology. 2005. PMID: 15841445
-
Quantitative Proteomics Analysis of the Hepatitis C Virus Replicon High-Permissive and Low-Permissive Cell Lines.PLoS One. 2015 Nov 6;10(11):e0142082. doi: 10.1371/journal.pone.0142082. eCollection 2015. PLoS One. 2015. PMID: 26544179 Free PMC article.
-
Eradication of hepatitis C virus subgenomic replicon by interferon results in aberrant retinol-related protein expression.Acta Med Okayama. 2012;66(6):461-8. doi: 10.18926/AMO/49042. Acta Med Okayama. 2012. PMID: 23254580
-
The proteins of the Hepatitis C virus: their features and interactions with intracellular protein phosphorylation.Arch Virol. 2003 Jul;148(7):1247-67. doi: 10.1007/s00705-003-0115-8. Arch Virol. 2003. PMID: 12827459 Review.
Cited by
-
Advances in proteomics data analysis and display using an accurate mass and time tag approach.Mass Spectrom Rev. 2006 May-Jun;25(3):450-82. doi: 10.1002/mas.20071. Mass Spectrom Rev. 2006. PMID: 16429408 Free PMC article. Review.
-
Activity-based protein profiling of the hepatitis C virus replication in Huh-7 hepatoma cells using a non-directed active site probe.Proteome Sci. 2010 Feb 4;8:5. doi: 10.1186/1477-5956-8-5. Proteome Sci. 2010. PMID: 20181094 Free PMC article.
-
Contribution of laser microdissection-based technology to proteomic analysis in hepatocellular carcinoma developing on cirrhosis.Proteomics Clin Appl. 2007 Jun;1(6):545-54. doi: 10.1002/prca.200600474. Epub 2007 May 11. Proteomics Clin Appl. 2007. PMID: 21136705 Free PMC article.
-
Clinical proteomics for liver disease: a promising approach for discovery of novel biomarkers.Proteome Sci. 2010 Dec 31;8:70. doi: 10.1186/1477-5956-8-70. Proteome Sci. 2010. PMID: 21192835 Free PMC article.
-
Proteomic approaches to analyzing hepatitis C virus biology.Proteomics. 2015 Jun;15(12):2051-65. doi: 10.1002/pmic.201500009. Epub 2015 May 5. Proteomics. 2015. PMID: 25809442 Free PMC article. Review.
References
-
- Adinolfi, L. E., M. Gambardella, A. Andreana, M. F. Tripodi, R. Utili, and G. Ruggiero. 2001. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 33:1358-1364. - PubMed
-
- Barba, G., F. Harper, T. Harada, M. Kohara, S. Goulinet, Y. Matsuura, G. Eder, Z. Schaff, M. J. Chapman, T. Miyamura, and C. Bréchot. 1997. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc. Natl. Acad. Sci. USA 94:1200-1205. - PMC - PubMed
-
- Bartenschlager, R., and V. Lohmann. 2000. Replication of hepatitis C virus. J. Gen. Virol. 81:1631-1648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

