Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway
- PMID: 15919913
- PMCID: PMC1143659
- DOI: 10.1128/JVI.79.12.7609-7616.2005
Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway
Abstract
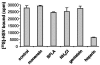
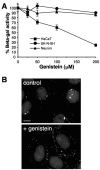
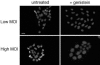
Herpes simplex virus (HSV) enters some laboratory cell lines via a pH-dependent, endocytic mechanism. We investigated whether this entry pathway is used in human cell types relevant to pathogenesis. Three different classes of lysosomotropic agents, which raise endosomal pH, blocked HSV entry into primary and transformed human keratinocytes, but not into human neurons or neuroblastoma lines. In keratinocytes, incoming HSV particles colocalized with markers of endocytic uptake. Treatment with the isoflavone genistein, an inhibitor of protein tyrosine kinases, reduced the delivery of incoming viral particles to the nuclear periphery and virus-induced gene expression in keratinocytes but not neurons. Moreover, in keratinocyte monolayer islets, HSV infected both the inner and outer cells in a genistein-sensitive manner, suggesting viral endocytosis from both basolateral and apical plasma membrane surfaces. Together, the results indicate that HSV enters human epidermal keratinocytes, but not neurons, by a low-pH, endocytic pathway that is dependent on host tyrosine phosphorylation. Thus, HSV utilizes fundamentally different cellular entry pathways to infect important target cell populations.
Figures



References
-
- Bodaghi, B., M. E. Slobbe-van Drunen, A. Topilko, E. Perret, R. C. Vossen, M. C. van Dam-Mieras, D. Zipeto, J. L. Virelizier, P. LeHoang, C. A. Bruggeman, and S. Michelson. 1999. Entry of human cytomegalovirus into retinal pigment epithelial and endothelial cells by endocytosis. Investig. Ophthalmol. Vis. Sci. 40:2598-2607. - PubMed
-
- Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82:1419-1422. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

