Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239
- PMID: 15919923
- PMCID: PMC1143664
- DOI: 10.1128/JVI.79.12.7707-7720.2005
Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239
Abstract
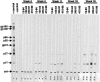
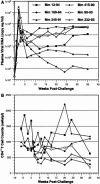
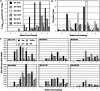
Genetically engineered simian immunodeficiency viruses (SIV) that is limited to a single cycle of infection was evaluated as a nonreplicating AIDS vaccine approach for rhesus macaques. Four Mamu-A*01(+) macaques were inoculated intravenously with three concentrated doses of single-cycle SIV (scSIV). Each dose consisted of a mixture of approximately equivalent amounts of scSIV strains expressing the SIV(mac)239 and SIV(mac)316 envelope glycoproteins with mutations in nef that prevent major histocompatibility complex (MHC) class I downregulation. Viral loads in plasma peaked between 10(4) and 10(5) RNA copies/ml on day 4 after the first inoculation and then steadily declined to undetectable levels over the next 4 weeks. SIV Gag-specific T-cell responses were detected in peripheral blood by MHC class I tetramer staining (peak, 0.07 to 0.2% CD8(+) T cells at week 2) and gamma interferon (IFN-gamma) enzyme-linked immunospot (ELISPOT) assays (peak, 50 to 250 spot forming cells/10(6) peripheral blood mononuclear cell at week 3). Following the second and third inoculations at weeks 8 and 33, respectively, viral loads in plasma peaked between 10(2) and 10(4) RNA copies/ml on day 2 and were cleared over a 1-week period. T-cell-proliferative responses and antibodies to SIV were also observed after the second inoculation. Six weeks after the third dose, each animal was challenged intravenously with SIV(mac)239. All four animals became infected. However, three of the four scSIV-immunized animals exhibited 1 to 3 log reductions in acute-phase plasma viral loads relative to two Mamu-A*01(+) control animals. Additionally, two of these animals were able to contain their viral loads below 2,000 RNA copies/ml as late as 35 weeks into the chronic phase of infection. Given the extraordinary difficulty in protecting against SIV(mac)239, these results are encouraging and support further evaluation of lentiviruses that are limited to a single cycle of infection as a preclinical AIDS vaccine approach.
Figures


Similar articles
-
Immunization with single-cycle SIV significantly reduces viral loads after an intravenous challenge with SIV(mac)239.PLoS Pathog. 2009 Jan;5(1):e1000272. doi: 10.1371/journal.ppat.1000272. Epub 2009 Jan 23. PLoS Pathog. 2009. PMID: 19165322 Free PMC article.
-
Mamu-B*17+ Rhesus Macaques Vaccinated with env, vif, and nef Manifest Early Control of SIVmac239 Replication.J Virol. 2018 Jul 31;92(16):e00690-18. doi: 10.1128/JVI.00690-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29875239 Free PMC article.
-
Vaccine protection against simian immunodeficiency virus in monkeys using recombinant gamma-2 herpesvirus.J Virol. 2011 Dec;85(23):12708-20. doi: 10.1128/JVI.00865-11. Epub 2011 Sep 7. J Virol. 2011. PMID: 21900170 Free PMC article.
-
A 30-year journey of trial and error towards a tolerogenic AIDS vaccine.Arch Virol. 2018 Aug;163(8):2025-2031. doi: 10.1007/s00705-018-3936-1. Epub 2018 Jul 24. Arch Virol. 2018. PMID: 30043201 Free PMC article. Review.
-
Evidence of a tolerogenic vaccine against AIDS in the Chinese macaque prefigures a potential human vaccine.Arch Virol. 2021 May;166(5):1273-1282. doi: 10.1007/s00705-020-04935-6. Epub 2021 Jan 28. Arch Virol. 2021. PMID: 33507389 Free PMC article. Review.
Cited by
-
Immunization with single-cycle SIV significantly reduces viral loads after an intravenous challenge with SIV(mac)239.PLoS Pathog. 2009 Jan;5(1):e1000272. doi: 10.1371/journal.ppat.1000272. Epub 2009 Jan 23. PLoS Pathog. 2009. PMID: 19165322 Free PMC article.
-
Dendritic cell immunoreceptor is a new target for anti-AIDS drug development: identification of DCIR/HIV-1 inhibitors.PLoS One. 2013 Jul 9;8(7):e67873. doi: 10.1371/journal.pone.0067873. Print 2013. PLoS One. 2013. PMID: 23874461 Free PMC article.
-
Generation and characterization of a defective HIV-1 Virus as an immunogen for a therapeutic vaccine.PLoS One. 2012;7(11):e48848. doi: 10.1371/journal.pone.0048848. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23144996 Free PMC article.
-
Two-dimensional gel-based approaches for the assessment of N-Linked and O-GlcNAc glycosylation in human and simian immunodeficiency viruses.Proteomics. 2008 Dec;8(23-24):4919-30. doi: 10.1002/pmic.200800608. Proteomics. 2008. PMID: 19072736 Free PMC article.
-
Understanding the failure of CD8+ T-cell vaccination against simian/human immunodeficiency virus.J Virol. 2007 Mar;81(6):2838-48. doi: 10.1128/JVI.01914-06. Epub 2007 Jan 3. J Virol. 2007. PMID: 17202215 Free PMC article.
References
-
- Alexander, L., R. S. Veazey, S. Czajak, M. DeMaria, M. Rosenzweig, A. A. Lackner, R. C. Desrosiers, and V. G. Sasseville. 1999. Recombinant simian immunodeficiency virus expressing green fluorescent protein identifies infected cells in rhesus monkeys. AIDS Res. Hum. Retrovir. 15:11-21. - PubMed
-
- Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. - PubMed
-
- Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 160:6062-6071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

