Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus
- PMID: 15919938
- PMCID: PMC1143678
- DOI: 10.1128/JVI.79.12.7845-7851.2005
Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus
Abstract
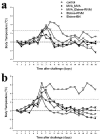
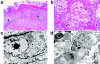
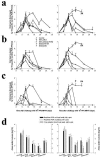
The use of classical smallpox vaccines based on vaccinia virus (VV) is associated with severe complications in both naive and immune individuals. Modified vaccinia virus Ankara (MVA), a highly attenuated replication-deficient strain of VV, has been proven to be safe in humans and immunocompromised animals, and its efficacy against smallpox is currently being addressed. Here we directly compare the efficacies of MVA alone and in combination with classical VV-based vaccines in a cynomolgus macaque monkeypox model. The MVA-based smallpox vaccine protected macaques against a lethal respiratory challenge with monkeypox virus and is therefore an important candidate for the protection of humans against smallpox.
Figures

Similar articles
-
Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox.Nature. 2004 Mar 11;428(6979):182-5. doi: 10.1038/nature02331. Nature. 2004. PMID: 15014500
-
Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge.J Infect Dis. 2005 Feb 1;191(3):372-81. doi: 10.1086/427265. Epub 2005 Jan 4. J Infect Dis. 2005. PMID: 15633096
-
Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model.Virology. 2005 Sep 1;339(2):164-75. doi: 10.1016/j.virol.2005.06.002. Virology. 2005. PMID: 15993917
-
Monitoring of human immunological responses to vaccinia virus.Methods Mol Biol. 2004;269:243-66. doi: 10.1385/1-59259-789-0:243. Methods Mol Biol. 2004. PMID: 15114020 Review.
-
Poxvirus Vaccines: Past, Present, and Future.Adv Exp Med Biol. 2024;1451:273-287. doi: 10.1007/978-3-031-57165-7_17. Adv Exp Med Biol. 2024. PMID: 38801584 Review.
Cited by
-
Current Insights into Diagnosis, Prevention Strategies, Treatment, Therapeutic Targets, and Challenges of Monkeypox (Mpox) Infections in Human Populations.Life (Basel). 2023 Jan 16;13(1):249. doi: 10.3390/life13010249. Life (Basel). 2023. PMID: 36676198 Free PMC article. Review.
-
Expression of CCL20 and granulocyte-macrophage colony-stimulating factor, but not Flt3-L, from modified vaccinia virus ankara enhances antiviral cellular and humoral immune responses.J Virol. 2006 Aug;80(15):7676-87. doi: 10.1128/JVI.02748-05. J Virol. 2006. PMID: 16840346 Free PMC article.
-
Smallpox antiviral drug development: satisfying the animal efficacy rule.Expert Rev Anti Infect Ther. 2006 Apr;4(2):277-89. doi: 10.1586/14787210.4.2.277. Expert Rev Anti Infect Ther. 2006. PMID: 16597208 Free PMC article. Review.
-
Vaccinia Virus: From Crude Smallpox Vaccines to Elaborate Viral Vector Vaccine Design.Biomedicines. 2021 Nov 26;9(12):1780. doi: 10.3390/biomedicines9121780. Biomedicines. 2021. PMID: 34944596 Free PMC article. Review.
-
Percutaneous Vaccination as an Effective Method of Delivery of MVA and MVA-Vectored Vaccines.PLoS One. 2016 Feb 19;11(2):e0149364. doi: 10.1371/journal.pone.0149364. eCollection 2016. PLoS One. 2016. PMID: 26895072 Free PMC article.
References
-
- Amorosa, V. K., and S. N. Isaacs. 2003. Separate worlds set to collide: smallpox, vaccinia virus vaccination, and human immunodeficiency virus and acquired immunodeficiency syndrome. Clin. Infect. Dis. 37:426-432. - PubMed
-
- Bray, M., and M. E. Wright. 2003. Progressive vaccinia. Clin. Infect. Dis. 36:766-774. - PubMed
-
- Breman, J. G., and I. Arita. 1980. The confirmation and maintenance of smallpox eradication. N. Engl. J. Med. 303:1263-1273. - PubMed
-
- Drexler, I., K. Heller, B. Wahren, V. Erfle, and G. Sutter. 1998. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J. Gen. Virol. 79:347-352. - PubMed
-
- Earl, P. L., J. L. Americo, L. S. Wyatt, L. A. Eller, J. C. Whitbeck, G. H. Cohen, R. J. Eisenberg, C. J. Hartmann, D. L. Jackson, D. A. Kulesh, M. J. Martinez, D. M. Miller, E. M. Mucker, J. D. Shamblin, S. H. Zwiers, J. W. Huggins, P. B. Jahrling, and B. Moss. 2004. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428:182-185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

