T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection
- PMID: 15919940
- PMCID: PMC1143651
- DOI: 10.1128/JVI.79.12.7860-7867.2005
T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection
Abstract
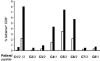
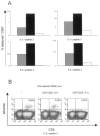
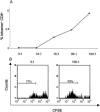
Chronic hepatitis C virus (HCV) infection is associated with impaired proliferative, cytokine, and cytotoxic effector functions of HCV-specific CD8(+) T cells that probably contribute significantly to viral persistence. Here, we investigated the potential role of T cells with a CD4(+)CD25(+) regulatory phenotype in suppressing virus-specific CD8(+) T-cell proliferation during chronic HCV infection. In vitro depletion studies and coculture experiments revealed that peptide specific proliferation as well as gamma interferon production of HCV-specific CD8(+) T cells were inhibited by CD4(+)CD25(+) T cells. This inhibition was dose dependent, required direct cell-cell contact, and was independent of interleukin-10 and transforming growth factor beta. Interestingly, the T-cell-mediated suppression in chronically HCV-infected patients was not restricted to HCV-specific CD8(+) T cells but also to influenza virus-specific CD8(+) T cells. Importantly, CD4(+)CD25(+) T cells from persons recovered from HCV infection and from healthy blood donors exhibited significantly less suppressor activity. Thus, the inhibition of virus-specific CD8(+) T-cell proliferation was enhanced in chronically HCV-infected patients. This was associated with a higher frequency of circulating CD4(+)CD25(+) cells observed in this patient group. Taken together, our results suggest that chronic HCV infection leads to the expansion of CD4(+)CD25(+) T cells that are able to suppress CD8(+) T-cell responses to different viral antigens. Our results further suggest that CD4(+)CD25(+) T cells may contribute to viral persistence in chronically HCV-infected patients and may be a target for immunotherapy of chronic hepatitis C.
Figures


References
-
- Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. - PubMed
-
- Bluestone, J. A., and A. K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3:253-257. - PubMed
-
- Cabrera, R., Z. Tu, Y. Xu, R. J. Firpi, H. R. Rosen, C. Liu, and D. R. Nelson. 2004. An immunomodulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology 40:1062-1071. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

