Inhibition of STAT 1 phosphorylation by human parainfluenza virus type 3 C protein
- PMID: 15919942
- PMCID: PMC1143680
- DOI: 10.1128/JVI.79.12.7877-7882.2005
Inhibition of STAT 1 phosphorylation by human parainfluenza virus type 3 C protein
Abstract
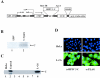
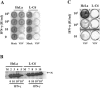
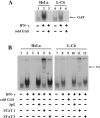
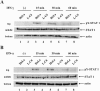
The P mRNA of the viruses belonging to the subfamily Paramyxovirinae possesses a unique property of giving rise to several accessory proteins by a process that involves the utilization of overlapping open reading frames (the C proteins) and by an "RNA-editing" mechanism (the V proteins). Although these proteins are considered accessory, numerous studies have highlighted the importance of these proteins in virus transcription and interferon signaling, including our previous observation on the role of human parainfluenza virus type 3 (HPIV 3) C protein in the transcription of viral genome (Malur et al., Virus Res. 99:199-204, 2004). In this report, we have addressed its role in interferon signaling by generating a stable cell line, L-C6, by using the lentiviral expression system which expresses HPIV 3 C protein. The L-C6 cells were efficient in abrogating both alpha and gamma interferon-induced antiviral states and demonstrated a drastic reduction in the formation of gamma-activated factor complexes in the cell extracts. Western blot analysis subsequently revealed a defect in the phosphorylation of STAT 1 in these cells. Taken together, our results indicate that HPIV 3 C protein is capable of counteracting the interferon signaling pathway by specifically inhibiting the activation of STAT 1.
Figures
References
-
- Bose, S., and A. K. Banerjee. 2003. Innate immune response against nonsegmented negative strand RNA viruses. J. Interferon Cytokine Res. 3:401-412. - PubMed
-
- Collins, P. L., R. M. Chanock, and K. McIntosh. 1996. Parainfluenza viruses, p. 1205-1241. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
-
- Delenda, C., G. Taylor, S. Hausmann, D. Garcin, and D. Kolakofsky. 1998. Sendai viruses with altered P, V, and W protein expression. Virology 242:327-337. - PubMed
-
- Durbin, A. P., J. M. McAuliffe, P. L. Collins, and B. R. Murphy. 1999. Mutations in the C, D and V open reading frames of human parainfluenza virus type 3 attenuate replication in rodents and primates. Virology 261:319-330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

