Investigation of the multimerization region of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) protein K-bZIP: the proposed leucine zipper region encodes a multimerization domain with an unusual structure
- PMID: 15919946
- PMCID: PMC1143620
- DOI: 10.1128/JVI.79.12.7905-7910.2005
Investigation of the multimerization region of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) protein K-bZIP: the proposed leucine zipper region encodes a multimerization domain with an unusual structure
Abstract
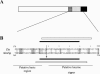
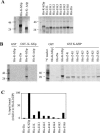
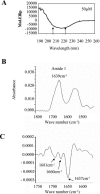
The K8 gene of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) shares many functional similarities with the BZLF1 gene of Epstein-Barr virus. The protein products of K8 and BZLF1, K-bZIP (RAP, K8) and Zta (BZLF1, ZEBRA, Z) have both been proposed to be members of the bZIP family of transcription factors, forming multimers via a coiled-coil motif termed a leucine zipper. Substantial evidence supporting this model for Zta is published. Here, we demonstrate that the proposed leucine zipper region of K-bZIP (amino acids 182 to 218) is required for multimer formation but that it does not fold as a coiled coil.
Figures

References
-
- Agre, P., P. F. Johnson, and S. L. McKnight. 1989. Cognate DNA binding specificity retained after leucine zipper exchange between GCN4 and C/EBP. Science 246:922-926. - PubMed
-
- Alber, T. 1992. Structure of the leucine zipper. Curr. Opin. Genet. Dev. 2:205-210. - PubMed
-
- AuCoin, D. P., K. S. Colletti, S. A. Cei, I. Papouskova, M. Tarrant, and G. S. Pari. 2004. Amplification of the Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 lytic origin of DNA replication is dependent upon a cis-acting AT-rich region and an ORF50 response element and the trans-acting factors ORF50 (K-Rta) and K8 (K-bZIP). Virology 318:542-555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

