Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress
- PMID: 15920153
- PMCID: PMC1602418
- DOI: 10.1016/S0002-9440(10)62478-9
Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress
Abstract
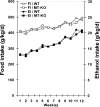
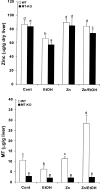
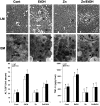
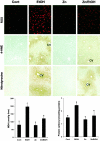
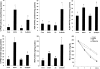
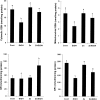
Alcoholic liver disease is associated with zinc decrease in the liver. Therefore, we examined whether dietary zinc supplementation could provide protection from alcoholic liver injury. Metallothionein-knockout and wild-type 129/Sv mice were pair-fed an ethanol-containing liquid diet for 12 weeks, and the effects of zinc supplementation on ethanol-induced liver injury were analyzed. Zinc supplementation attenuated ethanol-induced hepatic zinc depletion and liver injury as measured by histopathological and ultrastructural changes, serum alanine transferase activity, and hepatic tumor necrosis factor-alpha in both metallothionein-knockout and wild-type mice, indicating a metallothionein-independent zinc protection. Zinc supplementation inhibited accumulation of reactive oxygen species, as indicated by dihydroethidium fluorescence, and the consequent oxidative damage, as assessed by immunohistochemical detection of 4-hydroxynonenal and nitrotyrosine and quantitative analysis of malondialdehyde and protein carbonyl in the liver. Zinc supplementation suppressed ethanol-elevated cytochrome P450 2E1 activity but increased the activity of alcohol dehydrogenase in the liver, without affecting the rate of blood ethanol elimination. Zinc supplementation also prevented ethanol-induced decreases in glutathione concentration and glutathione peroxidase activity and increased glutathione reductase activity in the liver. In conclusion, zinc supplementation prevents alcoholic liver injury in an metallothionein-independent manner by inhibiting the generation of reactive oxygen species (P450 2E1) and enhancing the activity of antioxidant pathways.
Figures
References
-
- Kiilerich S, Dietrichson O, Loud FB, Naestoft J, Christoffersen P, Juhl E, Kjems G, Christiansen C. Zinc depletion in alcoholic liver diseases. Scand J Gastroenterol. 1980;15:363–367. - PubMed
-
- McClain CJ, Antonow DR, Cohen DA, Shedlofsky S. Zinc metabolism in alcoholic liver disease. Alcohol Clin Exp Res. 1986;10:582–589. - PubMed
-
- Bode JC, Hanisch P, Henning H, Koenig W, Richter FW, Bode C. Hepatic zinc content in patients with various stages of alcoholic liver disease and in patients with chronic active and chronic persistent hepatitis. Hepatology. 1988;8:1605–1609. - PubMed
-
- Dinsmore W, Callender ME, McMaster D, Todd SJ, Love AH. Zinc absorption in alcoholics using zinc-65. Digestion. 1985;32:238–242. - PubMed
-
- Valberg LS, Flanagan PR, Ghent CN, Chamberlain MJ. Zinc absorption and leukocyte zinc in alcoholic and nonalcoholic cirrhosis. Dig Dis Sci. 1985;30:329–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

