Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells
- PMID: 15920163
- PMCID: PMC1602425
- DOI: 10.1016/S0002-9440(10)62488-1
Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells
Abstract
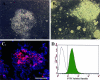
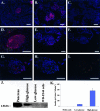
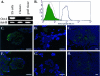
Embryonic stem (ES) cells have been proposed to be a powerful tool in the study of pancreatic disease, as well as a potential source for cell replacement therapy in the treatment of diabetes. However, data demonstrating the feasibility of using pancreatic islet-like cells differentiated from ES cells remain controversial. In this study we characterized ES cell-derived insulin-expressing cells and assessed their suitability for the treatment of type I diabetes. ES cell-derived insulin-stained cell clusters expressed insulin mRNA and transcription factors associated with pancreatic development. The majority of insulin-positive cells in the clusters also showed immunoreactivity for C-peptide. Insulin was stored in the cytoplasm and released into the culture medium in a glucose-dependent manner. When the cultured cells were transplanted into diabetic mice, they reversed the hyperglycemic state for approximately 3 weeks, but the rescue failed due to immature teratoma formation. Our studies demonstrate that reversal of hyperglycemia by transplantation of ES cell-derived insulin-producing cells is possible. However, the risk of teratoma formation would need to be eliminated before ES cell-based therapies for the treatment of diabetes are considered.
Figures



References
-
- Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259–268. - PubMed
-
- Lechner A, Leech CA, Abraham EJ, Nolan AL, Habener JF. Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cassette transporter. Biochem Biophys Res Commun. 2002;293:670–674. - PubMed
-
- Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes. 2004;53:2143–2152. - PubMed
-
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

