Dual infection with Helicobacter bilis and Helicobacter hepaticus in p-glycoprotein-deficient mdr1a-/- mice results in colitis that progresses to dysplasia
- PMID: 15920164
- PMCID: PMC1602406
- DOI: 10.1016/S0002-9440(10)62489-3
Dual infection with Helicobacter bilis and Helicobacter hepaticus in p-glycoprotein-deficient mdr1a-/- mice results in colitis that progresses to dysplasia
Abstract
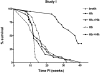
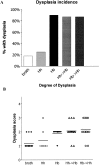
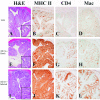
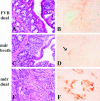
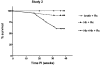
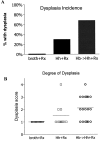
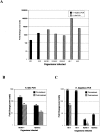
Patients with inflammatory bowel disease (IBD) are at increased risk for developing high-grade dysplasia and colorectal cancer. Animal IBD models that develop dysplasia and neoplasia may help elucidate the link between inflammation and colorectal cancer. Mdr1a-/- mice lack the membrane efflux pump p-glycoprotein and spontaneously develop IBD that can be modulated by infection with Helicobacter sp: H. bilis accelerates development of colitis while H. hepaticus delays disease. In this study, we determined if H. hepaticus infection could prevent H. bilis-induced colitis. Unexpectedly, a proportion of dual-infected mdr1a-/- mice showed IBD with foci of low- to high-grade dysplasia. A group of dual-infected mdr1a-/- animals were maintained long term (39 weeks) by intermittent feeding of medicated wafers to model chronic and relapsing disease. These mice showed a higher frequency of high-grade crypt dysplasia, including invasive adenocarcinoma, possibly because H. hepaticus, in delaying the development of colitis, allows time for transformation of epithelial cells. Colonic epithelial preparations from co-infected mice showed increased expression of c-myc (5- to 12-fold) and interleukin-1alpha/beta (600-fold) by real-time polymerase chain reaction relative to uninfected wild-type and mdr1a-/- animals. This animal model may have particular relevance to human IBD and colorectal cancer because certain human MDR1 polymorphisms have been linked to ulcerative colitis and increased risk for colorectal cancer.
Figures



References
-
- Dancourt V, Faivre J. Epidemiology and screening of colorectal cancer. Rev Prat. 2004;54:135–142. - PubMed
-
- Kobaek-Larsen M, Thorup I, Diederichsen A, Fenger C, Hoitinga MR. Review of colorectal cancer and its metastases in rodent models: comparative aspects with those in humans. Comp Med. 2000;50:16–26. - PubMed
-
- Galvez JJ, Cardiff RD, Munn RJ, Borowsky AD, Boivin GP, Groden J, Longnecker DS, Shmidt EN, Nikitin AY, Connolly DC, Hamilton TC. Mouse models of human cancers (part 2). Comp Med. 2004;54:13–28. - PubMed
-
- Karlen P, Lofberg R, Brostrom O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 1999;94:1047–1052. - PubMed
-
- Bachwich DR, Lichtenstein GR, Traber PG. Cancer in inflammatory bowel disease. Med Clin North Am. 1994;78:1399–1412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

