Differential expression of interleukin-8 and its receptors in the neuroendocrine and non-neuroendocrine compartments of prostate cancer
- PMID: 15920165
- PMCID: PMC1602414
- DOI: 10.1016/S0002-9440(10)62490-X
Differential expression of interleukin-8 and its receptors in the neuroendocrine and non-neuroendocrine compartments of prostate cancer
Abstract
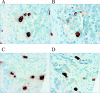
Hormonal therapy (androgen ablation and/or inhibition of androgen action) is the treatment of choice for advanced prostate cancer. After an initial response in most patients, tumors invariably progress to an androgen-independent state. It is unclear how prostate cancer cells proliferate without androgen. Recent studies suggest that interleukin-8 may promote androgen-independent proliferation, but the source of interleukin-8 in the prostate is unknown. Using immunohistochemistry, we show that interleukin-8 was expressed by the neuroendocrine tumor cells in human prostate cancer tissue. Expression of the interleukin-8 receptor CXCR1 was negative or low in benign prostatic tissue and was frequently increased in malignant cells of high-grade prostatic intraepithelial neoplasia and prostate cancer; however, CXCR1 was not detected in the neuroendocrine tumor cells, suggesting a paracrine mechanism by which interleukin-8 produced by neuroendocrine tumor cells stimulates androgen-independent proliferation of prostate cancer. Neuroendocrine tumor cells expressed another type of interleukin-8 receptor, CXCR2, suggesting an autocrine mechanism by which interleukin-8 regulates the differentiation or function of the neuroendocrine cells. These results, combined with previous reports that neuroendocrine differentiation is induced by hormonal therapy, suggest that neuroendocrine cells play an important role in promoting androgen-independent growth of prostate cancer through interleukin-8 signaling.
Figures




References
-
- Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. - PubMed
-
- Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–2898. - PubMed
-
- Balk SP. Androgen receptor as a target in androgen-independent prostate cancer. Urology. 2002;60:132–139. - PubMed
-
- Tan J, Sharief Y, Hamil KG, Gregory CW, Zang DY, Sar M, Gumerlock PH, DeVere White RW, Pretlow TG, Harris SE, Wilson EM, Mohler JL, French FS. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997;11:450–459. - PubMed
-
- Wilding G, Chen M, Gelmann EP. Aberrant response in vitro of hormone-responsive prostate cancer cells to antiandrogens. Prostate. 1989;14:103–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

