Anthrax lethal toxin induces endothelial barrier dysfunction
- PMID: 15920171
- PMCID: PMC1602427
- DOI: 10.1016/S0002-9440(10)62496-0
Anthrax lethal toxin induces endothelial barrier dysfunction
Abstract
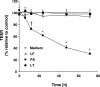
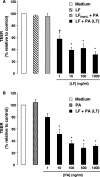
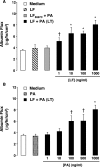
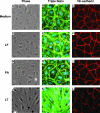
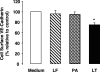
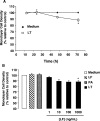
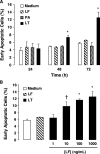
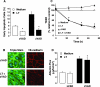
Hemorrhage and pleural effusion are prominent pathological features of systemic anthrax infection. We examined the effect of anthrax lethal toxin (LT), a major virulence factor of Bacillus anthracis, on the barrier function of primary human lung microvascular endothelial cells. We also examined the distribution patterns of cytoskeletal actin and vascular endothelial-cadherin (VE-cadherin), both of which are involved in barrier function regulation. Endothelial monolayers cultured on porous membrane inserts were treated with the LT components lethal factor (LF) and protective antigen (PA) individually, or in combination. LT induced a concentration- and time-dependent decrease in transendothelial electrical resistance that correlated with increased permeability to fluorescently labeled albumin. LT also produced a marked increase in central actin stress fibers and significantly altered VE-cadherin distribution as revealed by immunofluorescence microscopy and cell surface enzyme-linked immunosorbent assay. Treatment with LF, PA, or the combination of an inactive LF mutant and PA did not alter barrier function or the distribution of actin or VE-cadherin. LT-induced barrier dysfunction was not dependent on endothelial apoptosis or necrosis. The present findings support a possible role for LT-induced barrier dysfunction in the vascular permeability changes accompanying systemic anthrax infection.
Figures
Similar articles
-
Anthrax lethal toxin-mediated disruption of endothelial VE-cadherin is attenuated by inhibition of the Rho-associated kinase pathway.Toxins (Basel). 2011 Oct;3(10):1278-93. doi: 10.3390/toxins3101278. Epub 2011 Oct 20. Toxins (Basel). 2011. PMID: 22069696 Free PMC article.
-
Anthrax lethal toxin induces ketotifen-sensitive intradermal vascular leakage in certain inbred mice.Infect Immun. 2006 Feb;74(2):1266-72. doi: 10.1128/IAI.74.2.1266-1272.2006. Infect Immun. 2006. PMID: 16428776 Free PMC article.
-
Endothelial barrier stabilization by a cyclic tandem peptide targeting VE-cadherin transinteraction in vitro and in vivo.J Cell Sci. 2009 May 15;122(Pt 10):1616-25. doi: 10.1242/jcs.040212. J Cell Sci. 2009. PMID: 19420236
-
The effects of anthrax lethal toxin on host barrier function.Toxins (Basel). 2011 Jun;3(6):591-607. doi: 10.3390/toxins3060591. Epub 2011 Jun 14. Toxins (Basel). 2011. PMID: 22069727 Free PMC article. Review.
-
New insights into the functions of anthrax toxin.Expert Rev Mol Med. 2006 Apr 11;8(7):1-18. doi: 10.1017/S1462399406010714. Expert Rev Mol Med. 2006. PMID: 16608555 Review.
Cited by
-
Penetration of the blood-brain barrier by Bacillus anthracis requires the pXO1-encoded BslA protein.J Bacteriol. 2009 Dec;191(23):7165-73. doi: 10.1128/JB.00903-09. Epub 2009 Oct 9. J Bacteriol. 2009. PMID: 19820089 Free PMC article.
-
Very Early Blood Diffusion of the Active Lethal and Edema Factors of Bacillus anthracis After Intranasal Infection.J Infect Dis. 2020 Feb 3;221(4):660-667. doi: 10.1093/infdis/jiz497. J Infect Dis. 2020. PMID: 31574153 Free PMC article.
-
Asymmetric dimethylarginine levels in patients with cutaneous anthrax: a laboratory analysis.Ann Clin Microbiol Antimicrob. 2014 Mar 26;13:12. doi: 10.1186/1476-0711-13-12. Ann Clin Microbiol Antimicrob. 2014. PMID: 24669818 Free PMC article.
-
Gram-positive and gram-negative bacterial toxins in sepsis: a brief review.Virulence. 2014 Jan 1;5(1):213-8. doi: 10.4161/viru.27024. Epub 2013 Nov 5. Virulence. 2014. PMID: 24193365 Free PMC article. Review.
-
The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS).PLoS Pathog. 2009 May;5(5):e1000456. doi: 10.1371/journal.ppat.1000456. Epub 2009 May 29. PLoS Pathog. 2009. PMID: 19478875 Free PMC article.
References
-
- Leppla SH. The bifactorial Bacillus anthracis lethal and oedema toxins. Alouf JA, Freer J, editors. London: Academic Press,; Comprehensive Sourcebook of Bacterial Protein Toxins. 1999:pp 243–263.
-
- Moayeri M, Leppla SH. The roles of anthrax toxin in pathogenesis. Curr Opin Microbiol. 2004;7:19–24. - PubMed
-
- Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–671. - PubMed
-
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

